Fillable Printable Fed Register 2010 28390
Fillable Printable Fed Register 2010 28390

Fed Register 2010 28390

69037
Federal Register/Vol. 75, No. 217/Wednesday, November 10, 2010/Proposed Rules
utilizing approved Federal Equivalent
Methods.
3. How does this information relate to
the Proposed Rule—Ambient Ozone
Monitoring Regulations: Revisions to
Network Design Requirements?
On July 16, 2009, EPA published a
proposed rule (74 FR 34525) to revise
the ozone monitoring network design
requirements. EPA proposed to modify
minimum monitoring requirements in
urban areas, add new minimum
monitoring requirements in non-urban
areas, and to extend the length of the
required ozone monitoring season in
some states.
In its proposal, EPA used ambient
ozone monitoring data obtained from
monitors operating outside (i.e., before
and after) the current required ozone
monitoring season to assess whether
ambient ozone concentrations could
approach or exceed the level of the
primary (8-hour) National Ambient Air
Quality Standards (NAAQS) during
these periods when monitoring is not
currently required. EPA’s analysis
utilized data for the period 2004–2006,
representing data from approximately
530 monitors which were operated on a
year-round basis. These data were
analyzed for two indicators: (1) The
number of exceedences of the NAAQS
(i.e., daily maximum 8-hour ozone
averages above 0.075 ppm) in the
months falling outside the currently
required ozone monitoring season for
each area, and (2) occurrences of daily
maximum 8-hour ozone averages of at
least 0.060 ppm, representing a value of
80 percent of the 0.075 ppm NAAQS. In
the proposal, we noted that the
operation of ozone monitors during
such periods of time when ambient
levels reach at least 80 percent of the
NAAQS ensures that persons unusually
sensitive to ozone are alerted to the
occurrence of elevated ozone
concentrations in their area, and
protects against the potential for
undocumented NAAQS exceedances.
The availability of these additional data
support many objectives including more
comprehensive real-time air quality
reporting to the public, ozone
forecasting programs, and the
verification of real-time air quality
forecast models.
As EPA completes revised analyses to
support the upcoming ozone monitoring
final rule, certain patterns of out-of-
season elevated 8-hour average ozone
concentrations, which were not
recognizable during 2004–2006, have
become apparent in newer data. These
patterns include a greater frequency of
occurrences of daily maximum 8-hour
ozone averages of at least 0.060 ppm
before and after the currently required
ozone monitoring seasons for the
aforementioned states than was
observed in the 2004–2006 dataset.
Accordingly, EPA is making these
newer data available for the specific
states that have such patterns.
4. Where can I get this information?
All of the information can be obtained
through the Air Docket and at //
www.regulations.gov (see
ADDRESSES
section above for docket contact
information).
5. What issue is EPA taking comment
on?
EPA requests comment on the
interpretation of the newer ambient 8-
hour average ozone monitoring data for
the states of Colorado, Kansas, and Utah
in the context of determining the final
ozone monitoring season requirements
for these states. Specifically, do the
patterns of elevated 8-hour average
ozone concentrations that occurred both
before and after the current required
ozone monitoring seasons for these
states support the revised seasons
proposed in the July 16, 2009,
rulemaking for these states? Do these
patterns support alternative required
monitoring seasons different from what
was proposed in the July 16, 2009,
rulemaking for these states? Issues for
consideration with regard to Colorado,
Kansas, and Utah are whether the
current ozone season requirements
should be maintained, whether the
proposed changes to seasons should be
finalized as proposed or revised, and
whether changes should be made for
these states that were not originally
proposed in the July 2009 rule.
6. What should I consider as I prepare
my comments for EPA?
You may find the following
suggestions helpful for preparing your
comments:
1. Explain your views as clearly as
possible.
2. Describe any assumptions that you
used.
3. Provide any technical information
or data you used that support your
views.
4. Provide specific examples to
illustrate your concerns.
5. Offer alternatives.
6. Make sure to submit your
comments by the comment period
deadline identified.
7. To ensure proper receipt by EPA,
identify the appropriate docket
identification number in the subject line
on the first page of your response. It
would also be helpful if you provided
the name, date, and Federal Register
citation related to your comments.
7. Submitting Confidential Business
Information (CBI)
Do not submit information you are
claiming as CBI to EPA through //
www.regulations.gov or e-mail. Clearly
mark the part of the information that
you claim to be CBI. Information so
marked will not be disclosed except in
accordance with procedures set forth in
40 CFR part 2. For CBI information in
a disk or CD–ROM that you mail to EPA,
mark the outside of the disk or CD–ROM
as CBI and then identify electronically
within the disk or CD–ROM the specific
information that is claimed as CBI. In
addition to one complete version of the
comment that includes information
claimed as CBI, a copy of the comment
that does not contain the information
claimed as CBI must be submitted for
inclusion in the public docket.
List of Subjects in 40 CFR Part 58
Air pollution control, Environmental
protection, Intergovernmental relations,
Reporting and recordkeeping
requirements, Ambient air monitoring.
Dated: November 3, 2010.
Mary E. Henigin,
Acting Director, Office of Air Quality Planning
and Standards.
[FR Doc. 2010–28259 Filed 11–9–10; 8:45 am]
BILLING CODE 6560–50–P
DEPARTMENT OF HEALTH AND
HUMAN SERVICES
Centers for Medicare & Medicaid
Services
42 CFR Part 455
[CMS–6034–P]
RIN 0938–AQ19
Medicaid Program; Recovery Audit
Contractors
AGENCY
: Centers for Medicare &
Medicaid Services (CMS), HHS.
ACTION
: Proposed rule.
SUMMARY
: This proposed rule would
provide guidance to States related to
Federal/State funding of State start-up,
operation and maintenance costs of
Medicaid Recovery Audit Contractors
(Medicaid RACs) and the payment
methodology for State payments to
Medicaid RACs in accordance with
section 6411 of the Affordable Care Act.
In addition, this rule proposes
requirements for States to assure that
adequate appeal processes are in place
for providers to dispute adverse
VerDate Mar<15>2010 15:01 Nov 09, 2010Jkt 223001PO 00000Frm 00012Fmt 4702Sfmt 4702E:\FR\FM\10NOP1.SGM10NOP1
erowe on DSK5CLS3C1PROD with PROPOSALS-1

69038
Federal Register/Vol. 75, No. 217/Wednesday, November 10, 2010/Proposed Rules
determinations made by Medicaid
RACs. Finally, the rule proposes that
States and Medicaid RACs coordinate
with other contractors and entities
auditing Medicaid providers and with
State and Federal law enforcement
agencies.
DATES
: To be assured consideration,
comments must be received at one of
the addresses provided below, no later
than 5 p.m. on January 10, 2011.
ADDRESSES
: In commenting, please refer
to file code CMS–6034–P. Because of
staff and resource limitations, we cannot
accept comments by facsimile (FAX)
transmission.
You may submit comments in one of
four ways (please choose only one of the
ways listed):
1. Electronically. You may submit
electronic comments on this regulation
to //www.regulations.gov. Follow
the ‘‘Submit a comment’’ instructions.
2. By regular mail. You may mail
written comments to the following
address ONLY: Centers for Medicare &
Medicaid Services, Department of
Health and Human Services, Attention:
CMS–6034–P, P.O. Box 8016, Baltimore,
MD 21244–8016.
Please allow sufficient time for mailed
comments to be received before the
close of the comment period.
3. By express or overnight mail. You
may send written comments to the
following address ONLY: Centers for
Medicare & Medicaid Services,
Department of Health and Human
Services, Attention: CMS–6034–P, Mail
Stop C4–26–05, 7500 Security
Boulevard, Baltimore, MD 21244–1850.
4. By hand or courier. If you prefer,
you may deliver (by hand or courier)
your written comments before the close
of the comment period to either of the
following addresses:
a. For delivery in Washington, DC—
Centers for Medicare & Medicaid
Services, Department of Health and
Human Services, Room 445–G, Hubert
H. Humphrey Building, 200
Independence Avenue, SW.,
Washington, DC 20201.
(Because access to the interior of the
Hubert H. Humphrey Building is not
readily available to persons without
Federal government identification,
commenters are encouraged to leave
their comments in the CMS drop slots
located in the main lobby of the
building. A stamp-in clock is available
for persons wishing to retain a proof of
filing by stamping in and retaining an
extra copy of the comments being filed.)
b. For delivery in Baltimore, MD—
Centers for Medicare & Medicaid
Services, Department of Health and
Human Services, 7500 Security
Boulevard, Baltimore, MD 21244–1850.
If you intend to deliver your
comments to the Baltimore address,
please call telephone number (410) 786–
7195 in advance to schedule your
arrival with one of our staff members.
Comments mailed to the addresses
indicated as appropriate for hand or
courier delivery may be delayed and
received after the comment period.
FORFURTHERINFORMATIONCONTACT
:
Joanne Davis, (410) 786–5127.
SUPPLEMENTARYINFORMATION
:
Inspection of Public Comments: All
comments received before the close of
the comment period are available for
viewing by the public, including any
personally identifiable or confidential
business information that is included in
a comment. We post all comments
received before the close of the
comment period on the following Web
site as soon as possible after they have
been received: //
www.regulations.gov. Follow the search
instructions on that Web site to view
public comments.
Comments received timely will also
be available for public inspection as
they are received, generally beginning
approximately 3 weeks after publication
of a document, at the headquarters of
the Centers for Medicare & Medicaid
Services, 7500 Security Boulevard,
Baltimore, Maryland 21244, Monday
through Friday of each week from
8:30 a.m. to 4 p.m. To schedule an
appointment to view public comments,
phone 1–800–743–3951.
I. Background
A. Current Law
The Medicaid program is a
cooperative Federal/State program
designed to allow States to receive
matching funds from the Federal
government to finance medical
assistance to eligible low income
beneficiaries. Medicaid was enacted in
1965 by the passage of Title XIX of the
Social Security Act (the Act).
States may choose to participate in
the Medicaid program by submitting a
State plan for medical assistance that is
approved by the Secretary. Although
States are not required to participate in
the Medicaid program, all States, the
District of Columbia, and the territories
do participate. Once a State elects to
participate in the program, it is required
to comply with its State plan, as well as
the requirements imposed by the Act
and applicable Federal regulations.
CMS is the primary Federal agency
providing oversight of State Medicaid
activities and facilitating program
integrity efforts. Our administration of
the Medicaid program requires that we
expend billions of dollars in Federal
matching payments to States for
Medicaid expenditures. We also have an
obligation to prevent, identify, and
recover improper payments to
individuals, contractors, and
organizations.
In November 2009, the President
signed Executive Order (E.O.) 13520 in
an effort to reduce improper payments
by increasing transparency in
government and holding agencies
accountable for reducing improper
payments. On March 22, 2010, the
Office of Management and Budget
(OMB) issued guidance for agencies
regarding the implementation of E.O.
13520 entitled Part III to OMB Circular
A–123, Appendix C (Appendix C).
Appendix C outlines the responsibilities
of agencies, determines the programs
subject to E.O. 13520, defines
supplemental measures and targets for
high priority programs, and establishes
reporting requirements under E.O.
13520 and procedures to identify
entities with outstanding payments.
Section 6411 of the Patient Protection
and Affordable Care Act (Pub. L. 111–
148, enacted on March 23, 2010) (the
Affordable Care Act) requires States to
establish programs in which they would
contract with 1 or more Recovery Audit
Contractors (Medicaid RACs) by
December 31, 2010. The Medicaid RACs
would review Medicaid claims
submitted by providers of services for
which payment may be made under
section 1902(a) of the Act or a waiver of
the State plan. Medicaid RACs would
identify underpayments, and identify
and collect overpayments from
providers.
Section 6411(a)(1) of the Affordable
Care Act amends section 1902(a)(42) of
the Act to provide that ‘‘the State shall
establish a program under which the
State contracts (consistent with State
law and in the same manner as the
Secretary enters into contracts with
recovery audit contractors under section
1893(h) ***) with 1 or more recovery
audit contractors for the purpose of
identifying underpayments and
overpayments and recouping
overpayments ***’’ To offer context
for our proposed approach to the
Medicaid RAC program, we provide
background discussion on the Medicare
RAC program.
B. Medicare RACs
Medicare RACs are private entities
with which CMS contracts to identify
and collect improper payments made in
Medicare’s fee-for-service program.
Initially authorized by the Congress as
a 3-year demonstration program by the
Medicare Prescription Drug,
Improvement, and Modernization Act of
VerDate Mar<15>2010 15:01 Nov 09, 2010Jkt 223001PO 00000Frm 00013Fmt 4702Sfmt 4702E:\FR\FM\10NOP1.SGM10NOP1
erowe on DSK5CLS3C1PROD with PROPOSALS-1

69039
Federal Register/Vol. 75, No. 217/Wednesday, November 10, 2010/Proposed Rules
2003 (Pub. L. 108–173, enacted on
December 8, 2003) (MMA), Medicare
RACs were permanently authorized in
the Tax Relief and Health Care Act of
2006 (Pub. L. 109–432, enacted on
December 20, 2006) (TRHCA). The
TRHCA directed CMS to expand the
Medicare RAC program nationwide by
January 1, 2010.
During the Medicare RAC
demonstration period, we contracted
with RACs to review claims from
Medicare participating providers and
suppliers in New York, Florida,
California, Arizona, Massachusetts, and
South Carolina. From 2005 through
2008, the Medicare RACs identified and
collected or corrected over $1 billion in
improper payments. The majority, or 96
percent, of the improper payments were
overpayments, while the remaining 4
percent were underpayments. As a
result of the demonstrated cost
effectiveness of the Medicare RACs, the
TRHCA required CMS to implement a
nationwide Medicare RAC program.
In our evaluation of the Medicare
RAC demonstration, providers surveyed
identified to CMS a number of concerns
and processes needing improvement.
For example, Medicare RACs were
reportedly inconsistent in documenting
their ‘‘good cause’’ for reviewing a claim.
In addition, providers complained that
a lack of physician presence on
Medicare RAC staffs contributed to
Medicare claims incorrectly being
denied. As a result, we met with
stakeholders, including the provider
community, and made a number of
changes to improve the Medicare RAC
program. In the permanent Medicare
RAC program, we directed Medicare
RACs to consistently document their
‘‘good cause’’ for reviewing a claim. We
now require each Medicare RAC to hire
a physician Medical Director to oversee
the medical record review process;
assist nurses, therapists, and certified
coders upon request; manage quality
assurance procedures; and maintain
relationships with provider
associations.
Both the MMA and the TRHCA
authorized CMS to pay Medicare RACs
on a contingency fee basis. Currently,
we pay Medicare RACs a contingency
fee rate ranging between 9 and 12.50
percent. These contingency fees are not
initially fixed by CMS, but are
established by the contractors through a
bidding process with CMS. Providers
may appeal Medicare RAC
determinations through the established
Medicare appeals process. During the
demonstration period, Medicare RACs
were required to return contingency fees
if the claim determination was
overturned on the first level appeal.
However, Medicare RACs were entitled
to retain contingency fees if the
determination was overturned on
subsequent levels of appeal. In the
permanent Medicare RAC program, we
now require Medicare RACs to return
the contingency fee payment if the
determination is overturned at any stage
of the appeals process.
C. Existing State Contingency Fee
Contracts
There is precedent for State Medicaid
contingency fee contracts for purposes
of recovering Medicaid overpayments
subject to third party liability (TPL)
requirements. Section 1902(a)(25) of the
Act requires States to take all reasonable
measures to determine the legal liability
of third parties to pay for medical
assistance furnished to a Medicaid
recipient under the State plan. In
addition, several States currently
contract with contingency fee
contractors to recover Medicaid
overpayments unrelated to TPL. In a
memorandum to CMS’ Regional
Administrators dated November 7, 2002,
we revised our policy prohibiting
Federal financial participation (FFP) for
States to pay costs to contingency fee
contractors, unrelated to TPL. The
revised policy allows contingency fee
payments if the following conditions are
met: (1) The intent of the contingency
fee contract must be to produce savings
or recoveries in the Medicaid program;
(2) the savings upon which the
contingency fee payment is based must
be adequately defined and the
determination of fee payments
documented to CMS’s satisfaction.
D. Medicaid RACs
Section 6411(a) of the Affordable Care
Act amends and expands section
1902(a)(42) of the Act to require States
to establish programs by December 31,
2010, to contract with 1 or more
Medicaid RACs to audit Medicaid
claims and to identify underpayments
and overpayments. While States are
required to establish their Medicaid
RAC programs by December 31, 2010,
via the State plan amendment process,
such programs need not be
implemented by this date. Instead,
absent an exception, States must fully
implement their Medicaid RAC
programs by April 1, 2011. We solicit
comments on the proposed
implementation date. States would be
required to report to CMS certain
elements describing the effectiveness of
their Medicaid RAC programs. These
elements would include, but not be
limited to general program descriptors
(for example, contract periods of
performance, contractors’ names,) and
program metrics (for example, number
of audits conducted, recovery amounts,
number of cases referred for potential
fraud). To implement this provision, we
propose to add a new subpart F to 42
CFR part 455.
Medicaid RACs would review post-
payment claims for improper payments,
overpayments, as well as
underpayments consistent with State
laws and regulations. Medicaid RACs
are a supplemental approach to
Medicaid program integrity efforts
already underway to ensure that States
make proper payments to providers.
Medicaid RACs do not replace any
existing State program integrity or audit
initiatives or programs. States must
maintain their existing program
integrity efforts uninterrupted with
respect to levels of funding and activity.
Should we detect evidence of fraud,
waste, and abuse that goes unreported
by the Medicaid RACs, we would work
closely with States to identify focus
areas for Medicaid RACs to improve
their efficacy.
The Affordable Care Act requires all
States to establish Medicaid RAC
programs, subject to such exceptions
and requirements as the Secretary may
require. This provision enables CMS to
vary the Medicaid RAC program
requirements, or exempt a State from
establishing a Medicaid RAC program if
inconsistent with State law. For
example, we may exempt a State from
the requirement to pay Medicaid RACs
on a contingent basis for collecting
overpayments when State law expressly
prohibits contingency fee contracting.
However, some other fee structure could
be required under any such exception.
Similarly, some State legislatures
must enact legislation before amending
their State plans. Because the
establishment of a Medicaid RAC
program is accomplished by State plan
amendment (SPA), many State
legislatures will not have the
opportunity to convene and enact such
an amendment to their State plans prior
to December 31, 2010, those States
would need to submit justifications to
defer establishing Medicaid RAC
programs until after those State
legislatures have met. For States that
require a State legislative change
granting authority to establish a
Medicaid RAC program, a SPA should
be submitted indicating that the
Medicaid RAC program cannot be
established until legislative authority is
granted.
Finally, there may be circumstances,
unrelated to the examples above, where
a State would seek to be excepted from
some or all of the requirements of the
Medicaid RAC program. Accordingly,
VerDate Mar<15>2010 15:01 Nov 09, 2010Jkt 223001PO 00000Frm 00014Fmt 4702Sfmt 4702E:\FR\FM\10NOP1.SGM10NOP1
erowe on DSK5CLS3C1PROD with PROPOSALS-1

69040
Federal Register/Vol. 75, No. 217/Wednesday, November 10, 2010/Proposed Rules
we propose at §455.516 that States
seeking exceptions from contracting
with Medicaid RACs must submit to
CMS a written justification for the
request. We anticipate granting
complete Medicaid RAC program
exceptions rarely, and only under the
most compelling of circumstances.
Section 6411(a) of the Affordable Care
Act amends section 1902(a)(42) of the
Act, which requires States to make the
following assurances to CMS regarding
Medicaid RAC programs:
•Under section 1902(a)(42)(B)(ii)(I)
of the Act, payments shall be made to
a Medicaid RAC contractor under
contract with a State only from amounts
recovered. As discussed more fully
below, we interpret this to mean that
payments to Medicaid RACs may not
exceed the total amounts recovered.
Additionally, we interpret this to mean
that payments to contractors may not be
made based upon amounts merely
identified but not recovered, or amounts
that may initially be recovered but that
subsequently must be repaid due to
determinations made in appeals
proceedings.
The payment methodology
determination for States, as well as
when Medicaid RACs should be paid by
States for their work are separate, but
closely related issues. The distinction
between amounts recovered and
amounts identified has implications for
how States would structure and
administer payment agreements with
Medicaid RACs, as well as the timing of
Medicaid RACs’ receipt of payments.
The options below illustrate two ways
that States could structure payments,
though they are not exhaustive.
In option one, for example, State A
pays RAC B its fee when RAC B
identifies and recovers an overpayment.
If provider C appeals and wins at any
stage, RAC B would be required to
return any portion of the contingency
fee that corresponds to the amount of an
overpayment that is overturned on
appeal.
In a second option, State D
determines it would pay RAC E its
contingency fee at the point at which
the recovery amount is fully
adjudicated; that is, at the conclusion of
any and all appeals available to provider
F. At that point, State D would pay RAC
E a contingency fee based on the
amount recovered.
•Under section
1902(a)(42)(B)(ii)(II)(aa) of the Act,
payments to a Medicaid RAC contractor
shall be made on a contingent basis for
collecting overpayments from the
amounts recovered. We are aware that
the proposed Medicaid RAC program,
by virtue of the differences between the
Medicare and Medicaid programs,
would not operate identically to the
Medicare RAC program. Recognizing
that each State must tailor its Medicaid
RAC activities to the uniqueness of its
own State, we are not proposing to
prescribe a set contingency fee rate for
States. Instead, we are proposing certain
guidelines based upon section
1902(a)(42)(B) of the Act and our
experience with the Medicare RAC
program, but allowing States the
discretion to set their fees within those
guidelines.
The Medicaid RACs would contract
with States and territories to identify
and collect overpayments, and would be
paid on a contingency fee basis by the
States. In the Medicare RAC program,
CMS contracts with Medicare RACs to
identify and recover overpayments from
Medicare providers, and to identify and
pay underpayments to Medicare
providers. We recognize the differences
among States and territories when it
comes to the issue of coordinating with
RACs the collection of overpayments.
The statute requires Medicaid RACs to
collect overpayments. However, some
States may not be able to delegate the
collection of overpayments to
contractors, while other States may have
other restrictions. In keeping with the
statutory language that States must
establish RAC programs consistent with
State law, we propose to provide States
with the flexibility of coordinating RAC
collections of overpayments.
Currently, there are 4 Medicare RAC
contractors operating. Those RACs are
paid an average contingency fee rate of
10.86 percent by CMS, with the highest
rate being 12.50 percent. We interpret
the statutory language that States must
establish a Medicaid RAC program ‘‘in
the same manner as the Secretary enters
into contracts with’’ Medicare RACs to
mean that some of the provisions of the
Medicare RAC program, generally,
should serve as a model for the
proposed Medicaid RAC program.
Accordingly, in §455.510(b)(3) and
(b)(4), we are proposing that we would
not provide Federal financial
participation (FFP) with respect to any
amount of a State’s contingency fee in
excess of the then highest Medicare
RAC contingency fee rate unless a State
requests an exception from CMS and
provides an acceptable justification.
In the absence of an approved
exception, a State may only pay a RAC
contractor, from the overpayments
collected, a contingency fee up to the
highest Medicare RAC contingency rate.
Any additional payment from the State
to the RAC must be made using State-
only funds. FFP is not available for
administrative expenditure claims for
the marginal difference between the
highest Medicare fee and the State’s
contingency fee. For example, unless an
exception applies, if the highest
Medicare RAC contingency fee is 12.50
percent and the State pays a Medicaid
RAC 14 percent, we would not pay the
Federal match on the 1.50 percent
difference. The State would use State-
only funds to make up the difference
between the State’s 14 percent
contingency fee and the 12.50 percent
contingency fee ceiling.
Currently, the Medicare RAC
contracts have an established period of
performance of up to 5 years, beginning
in 2009. Initially, the maximum
contingency rate for which FFP would
be available for States to pay Medicaid
RACs would be the highest Medicare
RAC contingency fee, which is 12.50
percent. That fee would be the
maximum rate when States implement
their RAC programs no later than April
1, 2011. Subsequently, we would make
States aware of any modifications to
payment methodology for contingency
fees and Medicaid RAC maximum
contingency rates for which FFP would
be available by publishing in a Federal
Register notice, by December 31, 2013,
the maximum Medicare contingency fee
rate, which would apply to FFP
availability for any Medicaid RAC
contracts covering the period of
performance beginning on July 1, 2014.
The established rate would be in place
for 5 years or until we publish a new
maximum rate in the Federal Register.
We solicit public comments on this
approach.
The Medicare RAC program is still a
relatively new program. We will apply
the lessons learned from the Medicare
RAC Demonstration, as well as from the
current program in providing States
technical support and assistance in their
efforts to implement their programs. For
example, States would require Medicaid
RACs to employ trained medical
professionals to review Medicaid
claims, as CMS now requires the
Medicare RACs to do. Additionally,
States may consider establishing
requirements regarding the
documentation of good cause to review
a claim. States should also be cognizant
of potential organizational conflicts of
interest, and should take affirmative
steps to identify and prevent any such
conflicts of interest.
The Office of the Inspector General of
U.S. Department of Health and Human
Services (HHS–OIG) recently reported
that the Medicare RACs identified over
$1 billion in improper payments, but
referred only two cases of potential
fraud to CMS. HHS–OIG opined that
Medicare RACs are disincentivized to
VerDate Mar<15>2010 15:01 Nov 09, 2010Jkt 223001PO 00000Frm 00015Fmt 4702Sfmt 4702E:\FR\FM\10NOP1.SGM10NOP1
erowe on DSK5CLS3C1PROD with PROPOSALS-1

69041
Federal Register/Vol. 75, No. 217/Wednesday, November 10, 2010/Proposed Rules
make referrals because the RACs receive
contingency fees. As we learn from the
lessons of Medicare RACs, we caution
States, in their design of Medicaid RAC
programs, to ensure that the Medicaid
RACs report instances of fraud and/or
criminal activity in addition to the
pursuit of overpayments. At
§455.508(b), we propose that whenever
RACs have reasonable grounds to
believe that fraud or criminal activity
has occurred, they must report it to the
appropriate law enforcement officials.
We solicit comments on these and other
issues that States should consider in the
design of their RAC programs. At
§455.508(c), we propose that Medicaid
RACs must meet the additional
requirements that States may establish.
•Under section
1902(a)(42)(B)(ii)(II)(bb) of the Act,
payment to a Medicaid RAC may be
made in the amounts as the State may
specify for identifying underpayments
from the amounts recovered. Currently,
Medicare RACs are paid a contingency
fee to identify underpayments, similar
to the way in which they are paid to
identify and recover overpayments.
With respect to Medicaid RACs, a State
may elect to use a similar approach, or
elect to establish a set fee or some other
fee structure for the identification of
underpayments. Consistent with a
State’s obligation to ensure that it pays
the right amount to the right provider
for the right service at the right time for
the right recipient, whatever
methodology a State chooses must
adequately incentivize the detection of
underpayments. In §455.510(c), we are
proposing to grant States the flexibility
to specify the underpayment fee for
Medicaid RACs. Additionally, we
would monitor the methodologies and
amounts paid by States to Medicaid
RACs to identify underpayments, and
may consider future additional
regulation depending on what data
reveals over time. We solicit public
comments on the proposal of allowing
States this flexibility.
The Affordable Care Act requires that
payments to a Medicaid RAC can only
come from amounts recovered. Federal
matching payments are not available for
RAC fees paid in excess of the
overpayment amounts collected. The
total fees paid to a Medicaid RAC
include both the amounts associated
with (1) identifying and recovering
overpayments; and (2) identifying
underpayments. Due to the Affordable
Care Act’s requirement that contingency
fees only come from amounts recovered,
total fees must not exceed the amounts
of overpayments collected.
Our experience with Medicare RAC
contractors is that overpayment
recoveries exceed underpayment
identification by more than a 9:1 ratio.
Therefore, it is not anticipated that
States would need to maintain a reserve
of recovered overpayments to fund
Medicaid RAC costs associated with
identifying underpayments. However,
States must maintain an accounting of
amounts recovered and paid. Further,
States must also ensure that they do not
pay in total RAC fees more than the total
amount of overpayments collected.
States must report overpayments to
CMS based on the net amount
remaining after all fees are paid to the
Medicaid RAC. Medicaid RACs may
only receive payments through the
contingency fee arrangement made in
accordance with these requirements and
the limitations discussed relating to the
maximum contingency fee amount. No
additional FFP is available for any other
State payment made to the RACs. This
treatment of the fees and expenditures
is directly linked to the specific
statutory language implementing
Medicaid RAC requirements. It does not
apply to Medicaid overpayment
recoveries in other contexts.
For example, RAC X’s fee for
overpayment identification is 10 percent
of the recovery amount. The fee for
identification of underpayments is 10
percent of the amount identified. If an
overpayment amount is $100, and the
total amount of underpayment is $20,
the total fees paid to the Medicaid RAC
would be $12 ($10 for the identification
of the overpayment and $2 for the
identification of the underpayment).
From the remaining amount of the $88
overpayment, the State would report,
and the Federal share of the identified
overpayment amount would be based
upon, the appropriate State match rate
for FFP. If the State pays a provider
based on the Medicaid RAC-identified
underpayment, and that expenditure is
claimed in accordance with timely filing
requirements, the $20 expenditure
would be matched at the regular FMAP,
or the appropriate FFP rate.
Currently, §433.312 requires States to
refund the Federal share of
overpayments, regardless of whether the
State actually recovers the
overpayments from the provider. This
requirement, and all other requirements
relating to overpayments, would apply
to Medicaid RAC identified
overpayments. Therefore, if a Medicaid
RAC identifies an overpayment to a
provider, the State is required to refund
the Federal share of the overpayment
amount to the Federal government net
of any contingency fee paid, as
discussed above.
•Under section 1902(a)(42)(B)(ii)(III)
of the Act, States must have an adequate
appeals process for entities to challenge
adverse Medicaid RAC determinations.
Each State already has in place an
administrative appeals infrastructure,
whereby a provider may avail itself of
its due process rights in an
administrative or judicial setting,
depending on State law or
administrative rule, with attendant
procedures for notice and an
opportunity to be heard. States may
utilize the existing appeals
infrastructure to adjudicate Medicaid
RAC appeals. States would be required
to submit to CMS a proposal describing
the appeals process, which must be
approved prior to implementing their
RAC programs.
Alternatively, a State may elect to
establish a separate appeals process for
RAC determinations, which must also
ensure providers adequate due process
in pursuing an appeal. Accordingly, at
§455.512 we propose to offer States the
flexibility to determine the appeals
process that would be available to
providers who seek review of adverse
RAC determinations.
Finally, it is important to note that the
potential length of a State’s
administrative appeals process may
have an impact on the methodology/
structure of the payment agreement
between a State and a Medicaid RAC.
For example, in a contract between State
X and RAC Y, where State X’s
administrative appeal process can
extend for 2 years, RAC Y may not
receive payment for an extended period
of time. Accordingly, RAC Y’s
contingency fee rate will most likely
reflect operating, maintenance and legal
costs over that period. Alternatively, in
State Z, completion of the
administrative appeals process takes 9
months. A contract between State Z and
RAC V may reflect a different
contingency fee rate.
•Under section
1902(a)(42)(B)(ii)(IV)(aa) of the Act, for
purposes of section 1903(a)(7) of the
Act, expenditures made by the State to
carry out the Medicaid RAC program are
necessary for the proper and efficient
administration of the State plan or
waiver of the plan. We interpret this
reference to section 1903(a)(7) of the Act
to mean that amounts expended by a
State to establish and operate the
Medicaid RAC program (aside from fee
payments, the treatment of which is
discussed elsewhere in this preamble)
are to be shared by the Federal
government at the 50 percent
administrative rate. We propose in
§455.514(b) that FFP would be available
to States for administrative costs subject
to reporting requirements.
VerDate Mar<15>2010 15:01 Nov 09, 2010Jkt 223001PO 00000Frm 00016Fmt 4702Sfmt 4702E:\FR\FM\10NOP1.SGM10NOP1
erowe on DSK5CLS3C1PROD with PROPOSALS-1

69042
Federal Register/Vol. 75, No. 217/Wednesday, November 10, 2010/Proposed Rules
•Section 1902(a)(42)(B)(ii)(IV)(bb)
and section 1903(d) of the Act applies
to amounts recovered (not merely
identified) under the Medicaid RAC
program. We propose that a State must
refund the Federal share of the net
amount of overpayment recoveries after
deducting a RAC’s fee payments (in
conformance with the restrictions
discussed above, including the
maximum allowed RAC contingency fee
and the exception process). In other
words, a State would take a RAC’s fee
payment ‘‘off the top’’ before calculating
the Federal share of the overpayment
recovery to be returned to CMS. Such
amounts recovered would be subject to
a State’s quarterly expenditure estimates
and the funding of the State’s share.
Additionally, we note that the
territories operate under a separate
funding authority that is statutorily-
capped. Because of the limitations
placed on FFP by section 1108(g) of the
Act, territories must assess the
feasibility of implementing and funding
Medicaid RAC contractors in their
jurisdictions. We would provide
technical assistance to the territories on
how to implement the provisions in
sections 1902(a)(42)(B)(ii)(I), (II), (III)
and (IV) of the Act. We solicit public
comment on the impact and feasibility
of such provisions on the territories.
•Under section
1902(a)(42)(B)(ii)(IV)(cc) of the Act,
States and their Medicaid RACs must
coordinate their efforts with other
contractors or entities performing audits
of entities receiving payments under the
State plan or waiver in the State,
including State and Federal law
enforcement agencies. We emphasize
that Medicaid RACs are not intended to,
and do not, replace any State program
integrity or audit initiatives or
programs. We propose in §455.508(b)
that an entity that wishes to enter a
contract with a State to perform the
functions of a Medicaid RAC must agree
to the coordination efforts.
Although overlapping or multiple
provider audits may be necessary, we
hope to minimize the likelihood of
overlapping audits. The Affordable Care
Act requires that States assure CMS that
they will coordinate Medicaid RAC
audit activity with an array of other
stakeholders that also conduct audits.
We anticipate working systematically,
both internally and with States. We
recognize that providers are currently
subject to audits by the States’ routine
program integrity audits, CMS’
Medicaid Integrity Contractors’ audits,
as well as audits conducted by other
State and Federal entities.
In addition to the obligation to
coordinate auditing efforts to reduce the
overburdening of Medicaid providers,
we also want to ensure coordination
between Medicaid RACs and law
enforcement organizations so that
suspected cases of fraud and abuse are
processed through the appropriate
channels. Law enforcement
organizations that may conduct audits
or investigations include, but are not
limited to, the HHS–OIG, the U.S.
Department of Justice, including the
Federal Bureau of Investigation, State
Medicaid Fraud Control Units, other
Federal and State law enforcement
agencies as appropriate and CMS. One
approach to ensure this coordination is
for States to establish Memoranda of
Understanding (MOUs) with their State
Medicaid Fraud Control Units (MFCUs),
program integrity units or other law
enforcement agencies. Nothing would
preclude a State from agreeing to pay
the Medicaid RAC a contingency fee
from funds ultimately recovered and
returned to the State as the State share
of an overpayment (or restitution) at the
close of the civil or criminal proceeding.
Finally, coordination may be a
challenge because of the number of
other agencies or entities that may be
conducting audits, but States are
obligated to ensure that Medicaid RACs
do not duplicate or compromise the
efforts of other entities performing
audits, including law enforcement that
may be investigating fraud and abuse.
II. Provisions of the Proposed
Regulations
In the section that follows, we discuss
the proposed changes to the regulations
in part 455 governing the Program
Integrity—Medicaid.
We propose to add a new ‘‘Subpart
F—Medicaid Recovery Audit
Contractors Program’’ that would
implement section 1902(a)(42)(B) of the
Act. Section 1902(a)(42)(B) sets forth
provisions relating to States establishing
recovery audit contractor programs in
which States will contract with 1 or
more Medicaid RACs to audit Medicaid
claims and to identify underpayments
and identify and recover overpayments.
We propose to add the following
sections:
A. Purpose (§455.500)
Proposed §455.500 sets forth the
purpose of the new subpart F. The
regulations would implement section
1902(a)(42)(B) of the Act that establishes
the Medicaid RAC program.
B. Establishment of Program (§455.502)
At proposed §455.502, we would
establish the Medicaid RAC program as
a measure for States to promote the
integrity of their Medicaid program, and
require that States enter into contracts
with one or more RACs to carry out the
activities described in §455.506, and
require that States report on certain
elements describing the effectiveness of
their Medicaid RAC program.
C. Definitions (§455.504)
We are proposing to define the
Medicaid RAC program as a recovery
audit contractor administered by a State
to identify overpayments and
underpayments and recoup
overpayments. We are proposing to
define the Medicare RAC program as a
recovery audit contractor program
administered by CMS to identify
overpayments and underpayments and
recoup overpayments.
D. Activities To Be Conducted by
Medicaid RACs (§455.506)
We propose at §455.506(a), to require
States to contract with one or more
RACs to engage in reviews of Medicaid
claims submitted by providers of
services or other individuals furnishing
items and services for which payment
has been made under section 1902(a) of
the Act to determine whether providers
have been underpaid or overpaid, and to
recover any overpayments identified.
We propose at §455.506(b), to leave to
the States’ discretion the manner in
which they will coordinate with
Medicaid RACs’ recoupment of
overpayments.
E. Eligibility Requirements for Medicaid
RACs (§455.508)
We propose at §455.508 to provide
that in order to be eligible to contract
with a State to perform the functions of
a Medicaid RAC, an entity must have
technical capability to carry out the
activities described in §455.506,
including employing trained medical
professionals to review Medicaid
claims. An entity must also agree to
coordinate with State and Federal
agencies, and meet any such other
requirements as the State may establish.
F. Payments to RACs (§455.510)
We propose at §455.510(a) that fees
paid to RACs shall be made only from
amounts recovered. We propose at
§455.510(b)(1) to require that the
contingency fee paid to Medicaid RACs
be based on a percentage of the
recovered overpayment amount. We
propose at §455.510(b)(2), that States
shall determine at what stage of the
audit process Medicaid RACs will
receive their contingency fee. We
propose at §455.510(b)(3) that, except as
provided in paragraph (b)(4), CMS will
not provide FFP for any amount of
contingency fee that exceeds the then
VerDate Mar<15>2010 15:01 Nov 09, 2010Jkt 223001PO 00000Frm 00017Fmt 4702Sfmt 4702E:\FR\FM\10NOP1.SGM10NOP1
erowe on DSK5CLS3C1PROD with PROPOSALS-1

69043
Federal Register/Vol. 75, No. 217/Wednesday, November 10, 2010/Proposed Rules
highest contingency fee rate paid to a
Medicare RAC. We propose at
§455.514(b)(4), that, on a case-by-case
basis, CMS will review and consider
substantially justified requests from
States to pay Medicaid RAC(s) a
contingency fee higher than the highest
Medicare RAC contingency fee. We
propose at §455.510(c) to require that
States determine the fee paid to
Medicaid RACs to identify
underpayments.
G. Medicaid RAC Provider Appeals
(§455.512)
We propose at §455.512 to require
States to provide a process for provider
appeals of adverse Medicaid RAC
determinations.
H. Federal Share of State Expense for
the Medicaid RAC Program (§455.514)
We propose at §455.514(a) that funds
expended by the State to carry out the
Medicaid RAC program shall be
considered necessary for the proper and
efficient administration of the State plan
or a waiver of the plan.
We propose at §455.514(a) that the
Federal share of State expense does not
include fees paid.
We propose at §455.514(b) that FFP is
available to States for administrative
costs of operation and maintenance of
Medicaid RACs, subject to CMS’
reporting requirements.
I. Exceptions From Medicaid RAC
Programs (§455.516)
We propose at §455.516, that States
that seek to be excepted from any of the
requirements of the Medicaid RAC
program must submit to CMS a written
justification for the request and get CMS
approval.
J. Applicability to the Territories
(§455.518)
We propose at §455.518 that the
provisions in §455.500 through
§455.516 are applicable to Guam,
Puerto Rico, U.S. Virgin Islands,
American Samoa and the
Commonwealth of the Northern Mariana
Islands.
III. Collection of Information
Requirements
Under the Paperwork Reduction Act
of 1995, we are required to provide 60-
day notice in the Federal Register and
solicit public comment before a
collection of information requirement is
submitted to the Office of Management
and Budget (OMB) for review and
approval. In order to fairly evaluate
whether an information collection
should be approved by OMB, section
3506(c)(2)(A) of the Paperwork
Reduction Act of 1995 requires that we
solicit comment on the following issues:
•The need for the information
collection and its usefulness in carrying
out the proper functions of our agency.
•The accuracy of our estimate of the
information collection burden.
•The quality, utility, and clarity of
the information to be collected.
•Recommendations to minimize the
information collection burden on the
affected public, including automated
collection techniques.
We are soliciting public comment on
each of these issues for the following
sections of this document that contain
information collection requirements
(ICRs):
A. ICRs Regarding State Submission of
Certain Elements Describing the
Effectiveness of Their Medicaid RAC
Programs (§455.502(c))
Section 455.502(c) would require
States to submit certain elements
describing the effectiveness of their
Medicaid RAC programs. These
elements will include, but not be
limited to general program descriptors
and program metrics evaluating
effectiveness. The burden associated
with this requirement is the time and
effort put forth by the State to aggregate
existing data that will be part of the
process of establishing their RAC
program. We estimate it would take 1
State 2 hours to perform this task. The
total annual burden for this requirement
is 112 hours.
B. ICRs Regarding State Justifications To
Pay Higher Contingency Fees
(§455.510(b)(4))
Section 455.510(b)(4) would require
States to submit justifications to CMS to
pay Medicaid RACs a contingency fee
higher than the highest Medicare RAC.
The burden associated with this
requirement is the time and effort put
forth by the State to prepare and submit
a justification. We estimate it would
take 1 State 60 hours to perform this
task. The total annual burden for this
requirement is 1680 hours.
C. ICRs Regarding Medicaid RAC
Provider Appeals (§455.512)
Section 455.512 would require States
to provide administrative appeal
procedures for Medicaid providers that
seek review of an adverse Medicaid
RAC determination.
The burden associated with this
requirement is the time and effort put
forth by the State to prepare and provide
administrative appeal procedures. We
estimate it would take 1 State 60 hours
to perform these tasks. The total annual
burden for this requirement is 3,360
hours.
D. ICRs Regarding Federal Share of
State Expense for the Medicaid RAC
Program (§455.514(b))
Section 455.514(b), FFP would be
available to States for the Federal share
of State expense for the Medicaid RAC
program subject to CMS’ reporting
requirements. The burden associated
with a State reporting quarterly
expenditure estimates is currently
approved under OMB# 0938–0067 with
an expiration date of August 31, 2011.
E. ICRs Regarding Exceptions From
Medicaid RAC Programs (§455.516)
Section 455.516 would require a State
that is seeking an exception from any of
the requirements of the Medicaid RAC
program to submit a written justification
to CMS.
The burden associated with this
requirement is the time and effort put
forth by the State to prepare and submit
a written justification for the request.
We estimate it would take 1 State 20
hours to meet this requirement. We
estimate approximately 15 States would
request an exception; therefore, the total
annual burden associated with this
requirement is 300 hours.
If you comment on these information
collection and recordkeeping
requirements, please do either of the
following:
1. Submit your comments
electronically as specified in the
ADDRESSES
section of this proposed rule;
or
2. Submit your comments to the
Office of Information and Regulatory
Affairs, Office of Management and
Budget, Attention: CMS Desk Officer,
[CMS–6034–P] Fax: (202) 395–6974; or
E-mail: OIRA_[email protected].
IV. Response to Comments
Because of the large number of public
comments we normally receive on
Federal Register documents, we are not
able to acknowledge or respond to them
individually. We will consider all
comments we receive by the date and
time specified in the
DATES
section of
this preamble, and, when we proceed
with a subsequent document, we will
respond to the comments in the
preamble to that document.
V. Regulatory Impact Analysis
A. Overall Impact
We have examined the impacts of this
rule as required by Executive Order
12866 on Regulatory Planning and
Review (September 30, 1993), the
Regulatory Flexibility Act (RFA)
(September 19, 1980, Pub. L. 96–354),
VerDate Mar<15>2010 15:01 Nov 09, 2010Jkt 223001PO 00000Frm 00018Fmt 4702Sfmt 4702E:\FR\FM\10NOP1.SGM10NOP1
erowe on DSK5CLS3C1PROD with PROPOSALS-1
69044
Federal Register/Vol. 75, No. 217/Wednesday, November 10, 2010/Proposed Rules
section 1102(b) of the Social Security
Act, section 202 of the Unfunded
Mandates Reform Act of 1995 (Pub. L.
104–4), Executive Order 13132 on
Federalism (August 4, 1999), and the
Congressional Review Act (5 U.S.C.
804(2)).
Executive Order 12866 directs
agencies to assess all costs and benefits
of available regulatory alternatives and,
if regulation is necessary, to select
regulatory approaches that maximize
net benefits (including potential
economic, environmental, public health
and safety effects, distributive impacts,
and equity). A regulatory impact
analysis (RIA) must be prepared for
major rules with economically
significant effects ($100 million or more
in any 1 year). We tentatively estimate
that this rulemaking may be
‘‘economically significant’’ as measured
by the $100 million threshold, and,
therefore, may be a major rule under the
Congressional Review Act.
This proposed rule applies to States’
requirement to contract with Medicaid
RACs to perform audits of Medicaid
providers on a contingency fee basis.
The majority of anticipated savings, as
a result of the provisions in this rule, are
related to improper payments. However,
as seen in the Medicare RAC
Demonstration period, we expect a
limited financial impact on most
providers, as significant improper
payments are relatively rare. The CMS
Office of the Actuary (OACT) estimated
the potential impact on Federal
Medicaid costs and savings. OACT used
the historical experience from the
Medicare program to estimate potential
savings to Medicaid. As such, these
estimates are highly uncertain, as a
result we offer estimates for FYs 2011
through 2015 to illustrate the potential
effects of this program. As a result,
OACTs estimates for FYs 2011 through
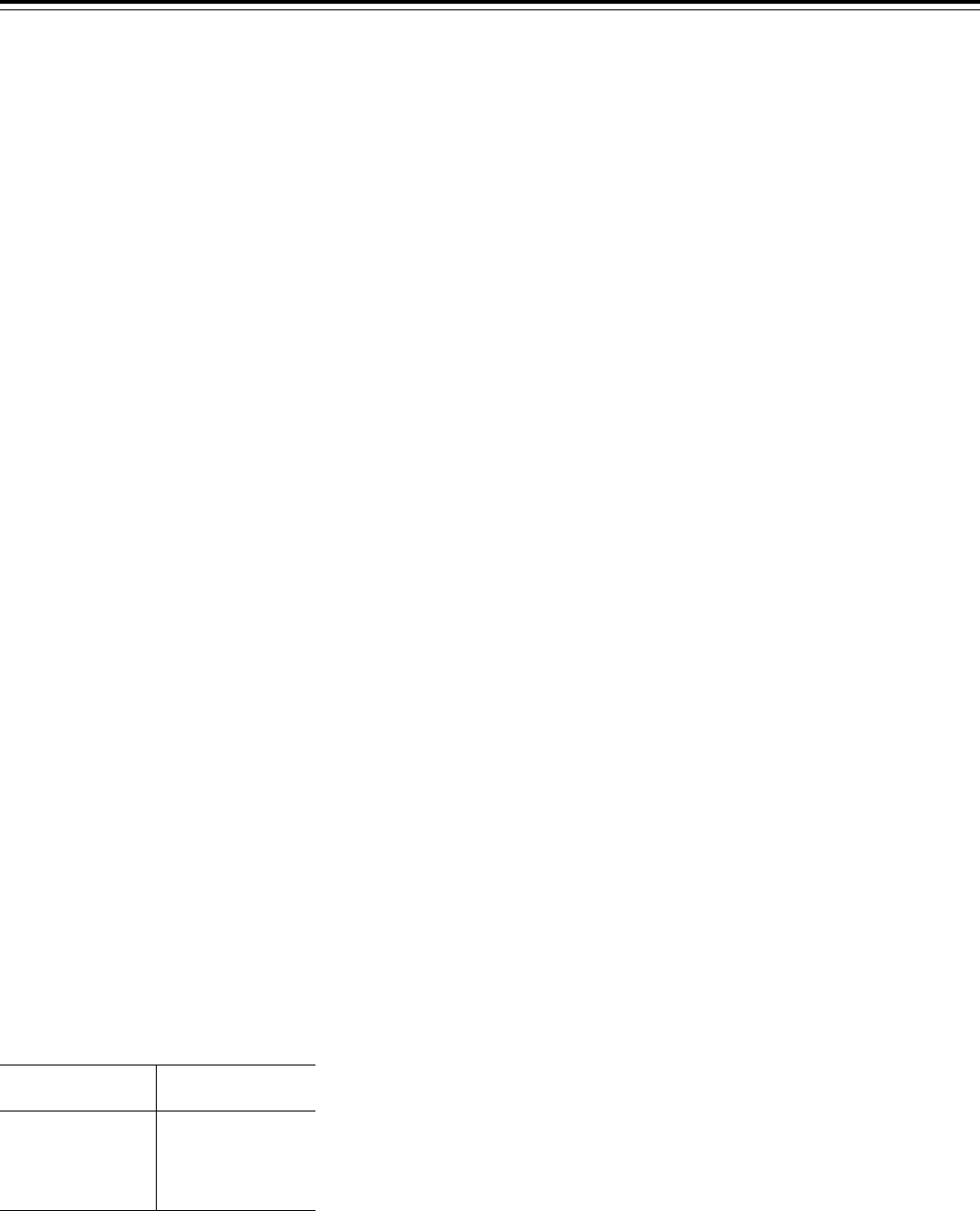
2015 are presented in Table A.
T
ABLE
A—P
OTENTIAL
N
ET
S
AVINGSTO
F
EDERAL
M
EDICAID
P
ROGRAMFROM
THE
E
XPANSIONOFTHE
R
ECOVERY
A
UDIT
C
ONTRACTOR
P
ROGRAM
Fiscal year
Estimated savings
(in millions of dollars)
2011..........................$80
2012..........................170
2013..........................250
2014..........................310
2015..........................330
We plan to refine the estimated
impacts in the final rule’s analysis and
we request comment on the potential
underpayments and overpayments
collected by States and the associated
contingency fees.
The RFA requires agencies to analyze
options for regulatory relief of small
businesses, if a rule has a significant
impact on a substantial number of small
entities. For purposes of the RFA, we
estimate that most Medicaid providers
are small entities as that term is used in
the RFA (include small businesses,
nonprofit organizations, and small
governmental jurisdictions). The great
majority of hospitals and most other
health care providers and suppliers are
small entities, either by being nonprofit
organizations or by meeting the SBA
definition of a small business (having
revenues of less than $7.0 million to
$34.5 million in any 1 year). For
purposes of the RFA, approximately 75
percent of Medicaid providers are
considered small businesses according
to the Small Business Administration’s
size standards with total revenues of
$35 million or less in any 1 year and 80
percent are nonprofit organizations.
Individuals and States are not included
in the definition of a small business
entity. Medicaid providers are required,
as a matter of course, to follow the
guidelines and procedures as specified
in State and Federal laws and
regulations. As such, Medicaid
providers must retain accurate billing
records for the requisite period of time.
Additionally, Medicaid providers must
cooperate in audits conducted by the
State and/or Federal governments and
their agents. Therefore, the Secretary
has determined that this proposed rule
will not have a significant economic
impact on a substantial number of small
entities.
In addition, section 1102(b) of the Act
requires us to prepare a regulatory
impact analysis if a rule may have a
significant impact on the operations of
a substantial number of small rural
hospitals. This analysis must conform to
the provisions of section 603 of the
RFA. For purposes of section 1102(b) of
the Act, we define a small rural hospital
as a hospital that is located outside of
a metropolitan statistical area and has
fewer than 100 beds. For the same
reason as stated above, this proposed
rule would not have a significant impact
on the operation of small rural
hospitals.
Section 202 of the Unfunded
Mandates Reform Act of 1995 (UMRA)
also requires that agencies assess
anticipated costs and benefits before
issuing any rule whose mandates
require spending in any 1 year of $100
million in 1995 dollars, updated
annually for inflation. In 2010, that
threshold is approximately $135
million. This proposed rule applies to
the States’ requirement to procure
Medicaid RACs to perform audits of
Medicaid providers on a contingency
fee basis. State expenditure associated
with this proposed rule would initially
involve directing or allocating personnel
resources to procurement activities. Per
the terms of the contracts, States would
not be expending funds over $135
million for RACs to perform the
contracts. Associated costs that may
include the operation of RAC programs,
collateral State personnel costs, and
maintenance of records are not expected
to exceed the $135 million threshold.
Therefore, this proposed rule is not
anticipated to have an effect on State,
local or tribal governments in the
aggregate, or by the private sector of
$135 million or more.
Executive Order 13132 establishes
certain requirements that an agency
must meet when it promulgates a
proposed rule (and subsequent final
rule) that imposes substantial direct
requirement costs on State and local
governments, preempts State law, or
otherwise has Federalism implications.
We have reviewed this proposed rule
under the threshold criteria of Executive
Order 13132, Federalism, and have
determined that it would not have
substantial direct effects on the rights,
roles, and responsibilities of States,
local or tribal governments.
B. Conclusion
We tentatively estimate that this rule
may be ‘‘economically significant’’ as
measured by the $100 million threshold
as set forth by Executive Order 12866,
as well as the Congressional Review
Act. The analysis above provides our
initial Regulatory Impact Analysis. We
have not prepared an analysis for
section 1102(b) of the RFA, section 202
of the UFMA and Executive Order
13132 because the provisions are not
impacted by this rule.
In accordance with the provisions of
Executive Order 12866, this regulation
was reviewed by the Office of
Management and Budget.
List of Subjects in 42 CFR Part 455
Fraud, Grant programs—health,
Health facilities, Health professions,
Investigations, Medicaid, Reporting and
recordkeeping requirements.
For the reasons set forth in the
preamble, the Centers for Medicare &
Medicaid Services proposes to amend
42 CFR chapter IV as set forth below:
PART 455—PROGRAM INTEGRITY—
MEDICAID
1. The authority citation for part 455
continues to read as follows:
VerDate Mar<15>2010 15:01 Nov 09, 2010Jkt 223001PO 00000Frm 00019Fmt 4702Sfmt 4702E:\FR\FM\10NOP1.SGM10NOP1
erowe on DSK5CLS3C1PROD with PROPOSALS-1

69045
Federal Register/Vol. 75, No. 217/Wednesday, November 10, 2010/Proposed Rules
Authority: Section 1102 of the Social
Security Act (42 U.S.C. 1302), section
1902(a)(42)(B) (42 U.S.C. 1396a(a)(42(B)).
2. New subpart F is added to read as
follows:
Subpart F—Medicaid Recovery Audit
Contractors Program
Sec.
455.500Purpose.
455.502Establishment of program.
455.504Definitions.
455.506Activities to be conducted by
Medicaid RACs.
455.508Eligibility requirements for
Medicaid RACs.
455.510Payments to RACs.
455.512Medicaid RAC provider appeals.
455.514Federal share of State expense for
the Medicaid RAC program.
455.516Exceptions from Medicaid RAC
program.
455.518Applicability to the territories.
Subpart F—Medicaid Recovery Audit
Contractors Program
§455.500Purpose.
This subpart implements section
1902(a)(42)(B) of the Social Security Act
that establishes the Medicaid Recovery
Audit Contractor (RAC) program.
§455.502Establishment of program.
(a) The Medicaid Recovery Audit
Contractor program (Medicaid RAC
program) is established as a measure for
States to promote the integrity of the
Medicaid program.
(b) States shall enter into contracts,
consistent with State law and in
accordance with this section, with
eligible Medicaid RACs to carry out the
activities described in §455.506 of this
subpart.
(c) States will be required to report to
CMS certain elements describing the
effectiveness of their Medicaid RAC
program.
§455.504Definitions.
As used in this subpart—
Medicaid RAC program means a
recovery audit contractor program
administered by a State to identify
overpayments and underpayments and
recoup overpayments.
Medicare RAC program means a
recovery audit contractor program
administered by CMS to identify
underpayments and overpayments and
recoup overpayments, established under
the authority of section 1893(h) of the
Act.
§455.506Activities to be conducted by
Medicaid RACs.
(a) Medicaid RACs will review claims
submitted by providers of items and
services or other individuals furnishing
items and services for which payment
has been made under section 1902(a) of
the Act or under any waiver of the State
plan to identify underpayments and
overpayments and recoup overpayments
for the States.
(b) States shall have the discretion to
coordinate with Medicaid RACs
regarding the recoupment of
overpayments.
§455.508Eligibility requirements for
Medicaid RACs.
An entity that wishes to perform the
functions of a Medicaid RAC may enter
into a contract with a State to carry out
any of the activities described in
§455.506 under the following
conditions:
(a) The entity shall demonstrate to a
State that it has the technical capability
to carry out the activities described in
§455.506 of this subpart. Evaluation of
technical capability must include the
employment of trained medical
professionals to review Medicaid
claims.
(b) In carrying out such activities, the
entity agrees to coordinate its efforts
with the State as well as the Office of
Inspector General of the U.S.
Department of Health and Human
Services, the U.S. Department of Justice,
including the Federal Bureau of
Investigation, State Medicaid Fraud
Control Units, other Federal and State
law enforcement agencies as appropriate
and CMS. Whenever the entity has
reasonable grounds to believe that fraud
or criminal activity has occurred, the
entity must report it immediately to
appropriate law enforcement officials.
(c) The Medicaid RAC meets such
other requirements as the State may
require.
§455.510Payments to RACs.
(a) General. Fees paid to RACs shall
be made only from amounts recovered.
(b) Overpayments. A State shall
determine the contingency fee rate to be
paid to a Medicaid RAC for the
identification and recovery of Medicaid
provider overpayments.
(1) The contingency fee paid to a
Medicaid RAC shall be based on a
percentage of the overpayment
recovered.
(2) States shall determine at what
stage in the Medicaid RAC process,
post-recovery, Medicaid RACs will
receive contingency fee payments.
(3) Except as provided in paragraph
(4) of this section, the contingency fee
may not exceed that of the highest
Medicare RAC, as specified by CMS in
the Federal Register, unless the State
submits, and CMS approves, a waiver of
the specified maximum rate. If a State
does not obtain a waiver of the specified
maximum rate, any amount exceeding
the specified maximum rate is not
eligible for Federal financial
participation (FFP), either from the
collected overpayment amounts, or in
the form of any other administrative or
medical assistance claimed expenditure.
(4) CMS will review and consider, on
a case-by-case basis, a State’s well-
justified request that CMS provide FFP
in paying a Medicaid RAC(s) a
contingency fee in excess of the then-
highest contingency fee paid to a
Medicare RAC.
(c) Underpayments. States shall
determine the fee paid to a Medicaid
RAC to identify underpayments.
§455.512Medicaid RAC provider appeals.
States shall provide appeal rights
available under State law or
administrative procedures to Medicaid
providers that seek review of an adverse
Medicaid RAC determination.
§455.514Federal share of State expense
of the Medicaid RAC program.
(a) Funds expended by the State for
the operation and maintenance of a
Medicaid RAC program, not including
fees paid to RACs, shall be considered
necessary for the proper and efficient
administration of the State plan or a
waiver of the plan.
(b) FFP is available to States for
administrative costs of operation and
maintenance of Medicaid RACs subject
to CMS’ reporting requirements.
§455.516Exceptions from Medicaid RAC
programs.
A State may seek to be excepted from
some or all Medicaid RAC contracting
requirements by submitting to CMS a
written justification for the request and
getting CMS approval.
§455.518Applicability to the territories.
The aforementioned provisions in
§455.500 through §455.516 of this
subpart are applicable to Guam, Puerto
Rico, U.S. Virgin Islands, American
Samoa, and the Commonwealth of the
Northern Mariana Islands.
Authority: (Catalog of Federal Domestic
Assistance Program No. 93.778, Medical
Assistance Program).
Dated: August 19, 2010.
Donald M. Berwick,
Administrator, Centers for Medicare &
Medicaid Services.
Approved: October 29, 2010.
Kathleen Sebelius,
Secretary, Health and Human Services.
[FR Doc. 2010–28390 Filed 11–5–10; 4:15 pm]
BILLING CODE 4120–01–P
VerDate Mar<15>2010 15:01 Nov 09, 2010Jkt 223001PO 00000Frm 00020Fmt 4702Sfmt 9990E:\FR\FM\10NOP1.SGM10NOP1
erowe on DSK5CLS3C1PROD with PROPOSALS-1



