Fillable Printable Drug Licence Application Form - UK
Fillable Printable Drug Licence Application Form - UK

Drug Licence Application Form - UK
1
MD29
DEPARTMENT OF HEALTH, SOCIAL SERVICES AND PUBLIC
SAFETY (DHSSPS)
MISUSE OF DRUGS ACT 1971
APPLICATION FOR LICENCES FOR THE POSSESSION, SUPPLY, MANUFACTURE AND
PRODUCTION OF CONTROLLED DRUGS
DETAILS OF LICENCE APPLICANT
1. Name of Business
2. Registered Business Number
Please provide a copy of the Certificate of Company Registration/Incorporation. If the business is not
registered, please provide reasons
3. Registered Business Address
Postcode:
4. Address of Premises for which a Licence is sought (If different from 3. above)
Postcode:
5. Other contact details
Telephone Number:
Email Address
Fax Number
Website
6. Is the Company/Organisation a Registered Charity? Yes/No
If Yes, please specify below your Charity Registration Number and registration date
7. Details of Managing Director/Person in Charge
Name
Title
Telephone Number
Email Address

2
Fax Number
Address (if different from 3 above)
Postcode:
8. Has any member of the company/organisation, member of its board and/or senior management
team ever been subject to legal business proceedings (administrative or financial), including
bankruptcy? Yes/No
9. If you have answered YES to above, please specify details below
10. Has any member of the company/organisation, members of its board and/or senior
management team ever been subject to any criminal convictions, including those relating to drugs?
Yes/No
11. If you have answered YES to above, please specify below details of convictions only. This
request does not extend to non-convictions, e.g. cautions warnings, reprimands etc, nor to spent
convictions under provisions in the Rehabilitation of Offenders Act 1974 (Exceptions) Order 1975.
12. Has the company/organisation ever been refused a licence from a central and/or local
government body or Issuing Authority, including the police? Yes/No
13. If the answer to 12 above is YES, please specify details below
14. Does the registered business comprise
(i) A limited company Yes/No
(ii) An individual Yes/No
(iii) A group of individuals (i.e. a partnership) Yes/No
(iv) A body corporate Yes/No
(v) None of the above Yes
15. If you have answered YES to 14(v) above, please specify details below
3
16. What is the business/trading style in regard to your licensing requirement?
(i) Pharmaceutical Manufacturer/Producer (delete as appropriate) Yes/No
(ii) Pharmaceutical Wholesaler Yes/No
(iii) Veterinary Wholesaler Yes/No
(iv) Healthcare Distributor Yes/No
(v) Product Packaging/labelling Yes/No
(vi) Importer Yes/No
(vii) Exporter Yes/No
(viii) Private Hospital/Treatment Centre/Clinic (delete as appropriate) Yes/No
(ix) Doctors Deputising Service Yes/No
(x) Drugs Research and Development Yes/No
(xi) Private forensic/Toxicology Service (delete as appropriate) Yes/No
(xii) None of the above Yes
17. If you have answered YES to 16(xii) above, please specify details below
18. Please state below the purposes(s) for which a licence/licences is/are being sought
19. If you have answered YES to 16(i) above, please specify details of all preparations to be
produced by virtue of the licence(s) for which application is made.
Drug Form Schedule Drug Form Schedule
20. If you have answered YES to 16(vi) and/or 16(vii) above, please specify details below:
Drug Form Schedule Quantity Import/Export Country
21. Please specify below the number and type of companies that will comprise your customer
base

4
22. Does the business possess a current MHRA (Department of Health) Wholesale Dealers
Licence or other MHRA licence(s)?
Yes/No
If you have answered YES, please specify details below
Licence No. Type of Licence Issue Date Expiry Date
23. Does the business possess a current HealthCare Commission/CSCI registration? Yes/No
If you have answered YES, please specify details below
Registration No. Issue Date Expiry Date
(You should be prepared to produce, upon request, your MHRA licence(s) and/or your
Registration documents.)
DETAILS OF PREMISES TO BE LICENSED
24. Are the premises to be licensed rented, leased or owned (Please tick appropriate box below)?
Rented Leased Owned
25. If the premises are rented or leased, please state below details regarding owner
Name of Owner:
Address:
Postcode:
Telephone number:
26. What is the total commercial value of controlled drug stock held in (a) Schedule 1 & 2; and (b)
Schedule 3 (Buprenorphine, Diethylpropion, Flunitrazepam and Temazepam only) Delete as necessary.
(a) (i) Up to £50,000 (b) (i) Up to £50,000
(ii) £50,000 - £500,000 (ii) £50,000 - £500,000
(iii) Over £500,000 (iii) Over £500,000
27. Do the premises have an electronic alarm system? Yes/No
28. If you answered yes is it (tick box)
NACOSS/SSAIB Registered
Redcare Connected
Police Unique Reference Number (URN)
Centrally Monitored
Annually Serviced
Level 1 or immediate police response
Separate zone alarm for controlled drug safe/store
5
29. Do the premises have:
(i) A CCTV system (centrally monitored *) Yes/No
(ii) Electronic stock recording system(s) Yes/No
(iii) Perimeter fencing Yes/No
(iv) Lockable physical security e.g. room, safe, cabinet Yes/No
(v) The attendance of site security guards Yes/No
* Please delete if not applicable
30. Please state below the details of the manager/person responsible for the security of the
premises to be licensed.
Name
Title
Telephone Number
Email Address
Fax Number
31. You need to have on site arrangements for the receipt, storage, assembly, picking, distribution,
recording and destruction of Controlled Drugs written down as a set of Standard Operating Procedures
(SOPs). Do you have these?
32. Please state below the details of the manager/person responsible for legal compliance and
regulatory affairs in respect of the premises to be licensed.
Name
Address
Postcode
Telephone Number
Email Address
Fax Number
Please note that the manager/responsible person will be expected to ensure that there is full compliance
with the statutory requirements of the Misuse of Drugs Act 1971, the Misuse of Drugs Regulations
(Northern Ireland) 2002, the stipulated conditions on any licence that may be issued and that there will
be in place written operating procedures that should accompany this application.
33. Is your ‘Responsible Person’ an employee or a consultant (please delete as appropriate)
34. Do you have the appropriate record keeping system in place? Yes/No
35 Please provide brief details of your record keeping

6
36. Do you require somebody within your company to be authorised to witness the destruction of
controlled drugs? Yes/No
37. Please provide details of the nominated authorised witness:
Name
Title
Telephone number
Email address
Fax number
Address*
line 2
line 3
postcode
SELECTION OF CONTROLLED DRUGS FOR INCLUSION ON LICENCE(S)
38. Please complete the attached Annex to this Application Form
DECLARATION UNDER MISUSE OF DRUGS ACT 1971
39. I/We hereby apply for the grant of DHSSPS drugs licence(s) in respect of those activities
included in this Application Form.
40. To the best of my/our knowledge and belief all the particulars I/we have declared in this
Application Form are correct and complete.
Signature(s):
Name(s) (BLOCK CAPITALS):
Position(s) held:
Date of signature:
7
APPLICATION FORM – GENERAL NOTES
41. Offences under Section 18(4)(a) of the Misuse of Drugs Act 1971. This Section of the Act
provides that a person commits an offence if, for the purposes of obtaining, whether for himself or
another, the issue or renewal of a licence under the Misuse of Drugs Act 1971, he makes any statement
or gives any information which he knows to be false in a material particular or recklessly gives any
information which is false. Being found guilty of this offence is punishable by fine and/or
imprisonment of up to 2 years.
42. Confidentiality. Information provided in this Application Form and attached ‘Drugs List’ will
be treated as Commercial-in-Confidence.
43. Signature(s) on Application Form. Applications will not be considered unless they bear the
signature of the applicant, a company partner or director, or the company secretary.
RETURN OF APPLICATION FORM/CONTROLLED DRUGS ANNEX
44. These documents should be returned to
Linda Hutcheson
Department of Health, Social Services and Public Safety
Pharmaceutical Advice & Services
Room D4.29
Castle Buildings, Stormont
BT4 3SQ
45. You should take a copy of the completed Application Form and Annex for your retention.
46. Where the Application Form and controlled drugs Annex do not provide sufficient space to
include all requested information, please provide typed supplementary sheets as appropriate.
FURTHER LICENSING INFORMATION/CONTACT POINTS
47. For further information and advice about licensing matters, including the completion of this
Application Form, you should contact Linda Hutcheson on:
Telephone No. 028 90 523274
Fax No. 028 90 522325
Email linda.hutcheson@dhsspsni.gov.uk
Department of Health, Social Services and Public Safety
Pharmaceutical Advice & Services
Room D4.29
Castle Buildings
Stormont
BT4 3SQ
8
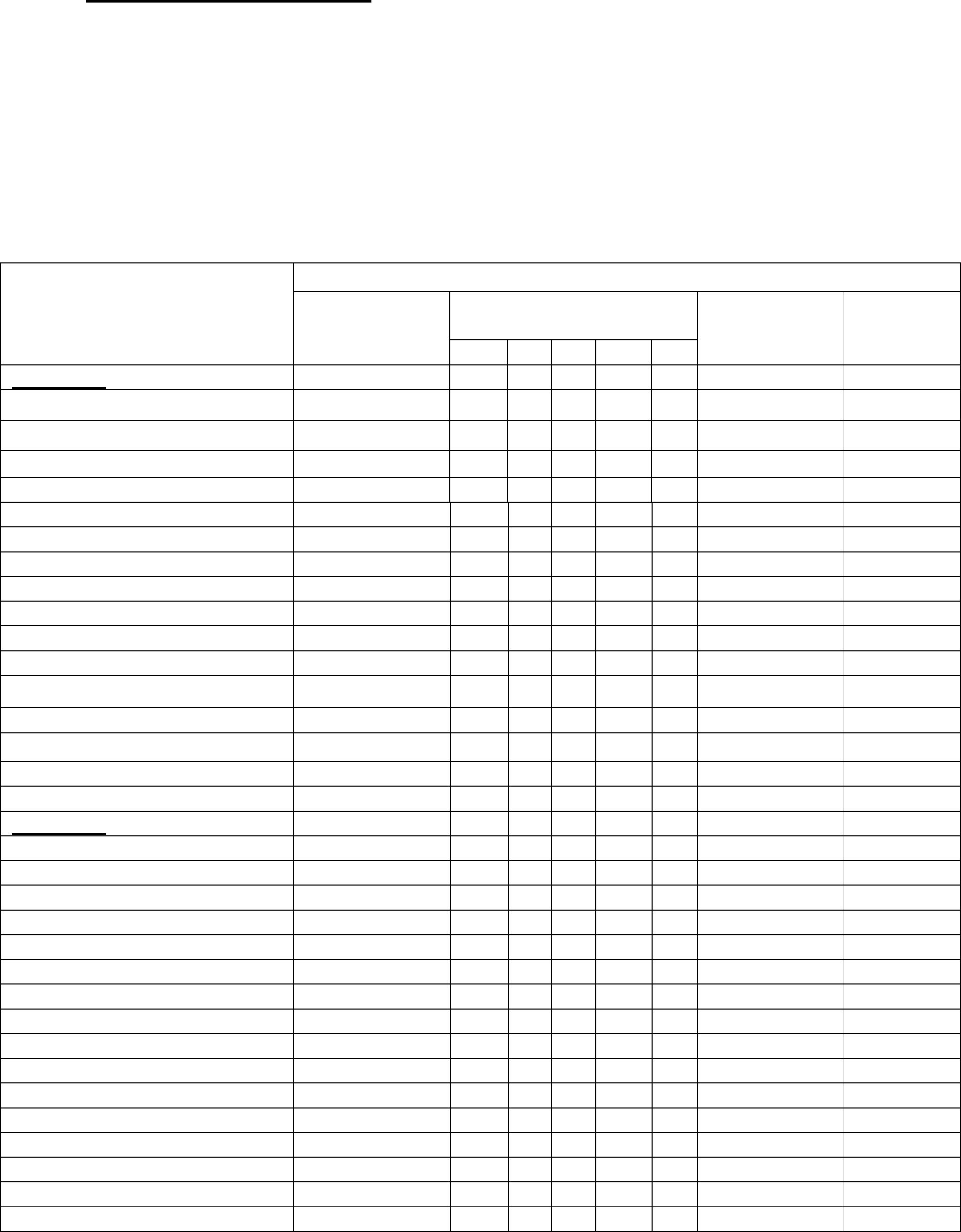
SELECTION OF CONTROLLED DRUGS AND TYPE OF LICENCES
REQUIRED
1. Please tick appropriately the boxes in the list below to indicate the
controlled drugs (CDs) and the type of licence(s) for which application
is being made.
2. The drugs listed below are those most commonly in use and have been
divided into their respective Schedules in accordance with the Misuse of
Drugs Legislation. Space has been left for the inclusion of drugs not
listed.
Names of Controlled Drugs
(CDs) in their respective
Schedules
TYPE OF LICENCE
To
manufacture
Base Drug(s)
To produce CDs in the
Schedules
To supply
and offer to
supply
To possess
only
1 2 3 4 5
Schedule 1
Cannabis
Cathinone
Coca Leaf
Concentrate of Poppy Straw
Ecstasy (MDMA)
Etryptamine
Lysergide
Mescaline
Methcathinone
Psilocin
Raw Opium
others (please specify)
Schedule 2
Alfentanil
Amphetamine
Cocaine
Codeine
Dextromoramide
Dextropropoxyphene
Diamorphine
Dihydrocodeine
Dihydroetorphine
Dipipanone
Ethlymorphine
Etorphine
Fentanyl
Hydromorphone
Medicinal Opium
Methadone
9
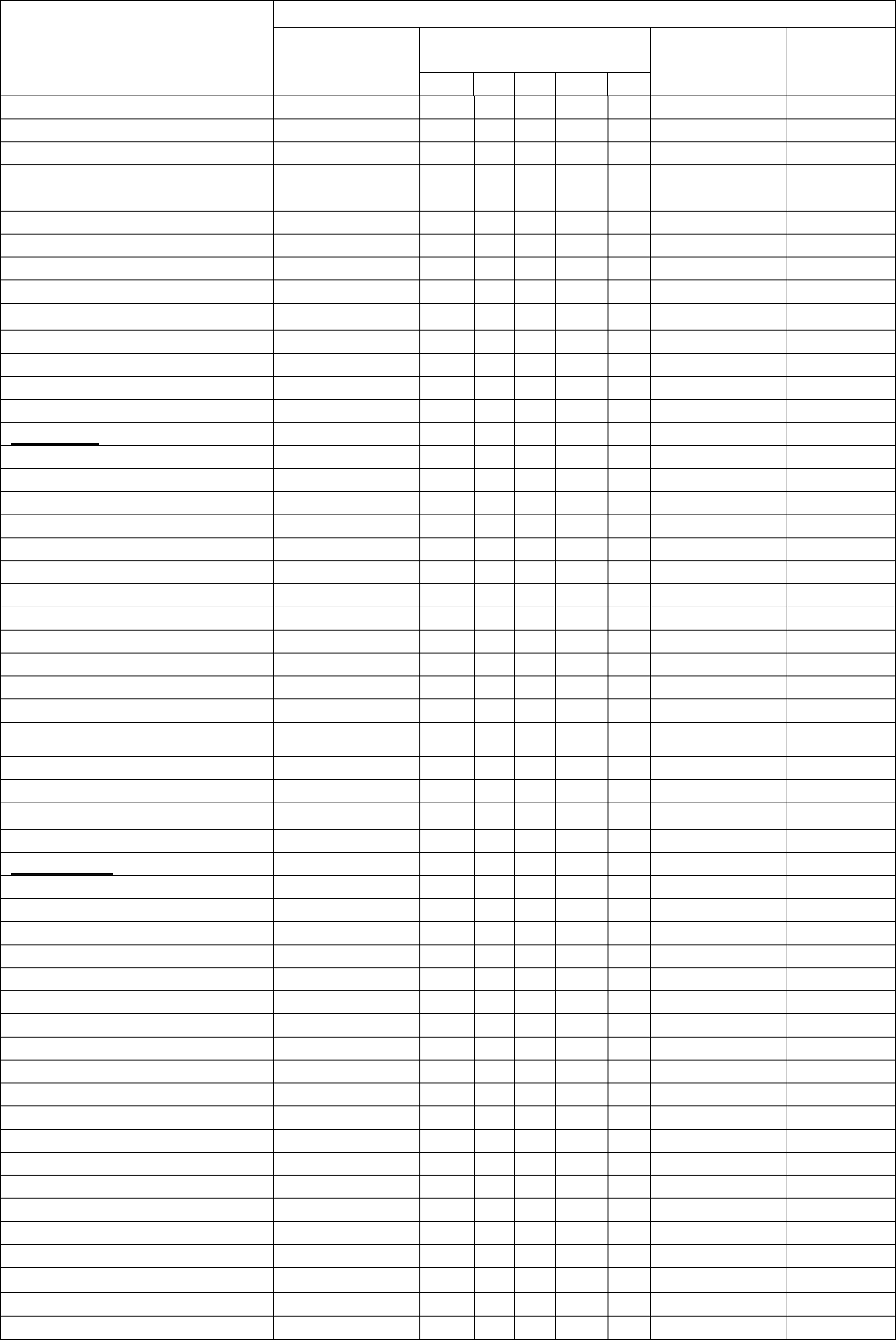
Names of Controlled Drugs
(CDs) in their respective
Schedules
TYPE OF LICENCE
To
manufacture
Base Drug(s)
To produce CDs in the
Schedules
To supply
and offer to
supply
To possess
only
1 2 3 4 5
Methylphenidate
Morphine (inc.papaveretum)
Oxycodone
Pethidine
Phenazocine
Phenmetrazine
Pholcodine
Remifentanil
Secobarbital
Others (please specify)
Schedule 3
Allobarbital
Amylobarbitone
Barbital
Buprenorphine
Butobarbital
Cathine
Cyclobarbital
Meprobamate
Pentazocine
Pentobarbital
Phenobarbitone
Temazepam
others (please specify)
Schedule 4.1
Alprazolam
Bromazepam
Clorazepate
Chlordiazepoxide
Clobazam
Clonazepam
Diazepam
Flurazepam
Loprazolam
Lorazepam
Lometazepam
Midazolam
Nitrazepam
Oxazepam
Triazolam
Zolpidem
others (please specify)
10
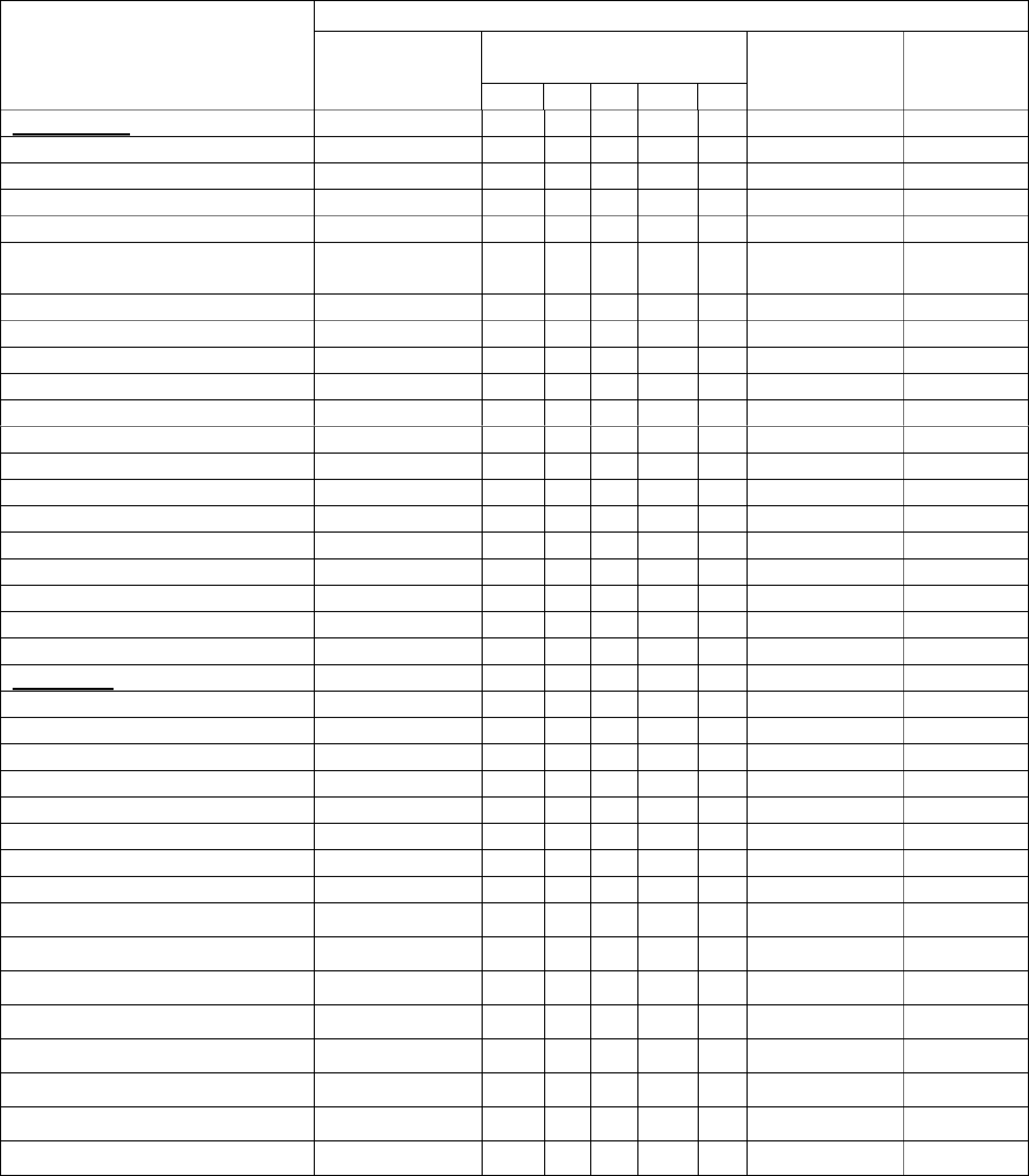
Names of Controlled Drugs
(CDs) in their respective
Schedules
TYPE OF LICENCE
To
manufacture
Base Drug(s)
To produce CDs in the
Schedules
To supply
and offer to
supply
To possess
only
1 2 3 4 5
Schedule 4.2
Chorionic gonadotrophin (HCG)
Clenbuterol
Gonadtrophin
Mesterolone
Non-human chorionic
gonadotrophin
Prasterone
Somatropin
Stanozolol
Testosterone
Trenbolone
others (please specify)
Schedule 5
Codeine
Dextropropoxyphene
Dihydrocodeine
Diphenoxylate
Morphine
Pholcodine
others (please specify)
Note:
No licence is required to "possess" Schedule 5 controlled drugs when in the
form of a medicinal product.