Fillable Printable 57.140 Uti Ltcf Blank
Fillable Printable 57.140 Uti Ltcf Blank
57.140 Uti Ltcf Blank
Form Approved
OMB No. 0920-0666
Exp. Date: 11/30/2019
www.cdc.gov/nhsn
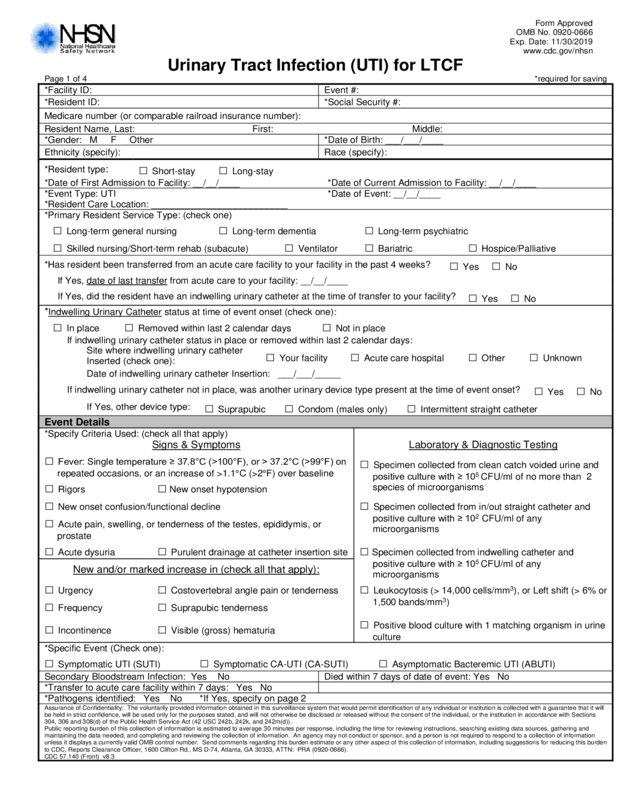
Urinary Tract Infection (UTI) for LTCF
Page 1 of 4 *required for saving
*Facility ID: Event #:
*Resident I D : *Social Security #:
Medicare number (or compar able railroad insurance number):
Resident Nam e, Last: First: Middle:
*Gender: M F Other *Date of Birth: ___/___/____
Ethnicity (specify): Race (specify):
*Resident type:
□ Short-stay □ Long-stay
*Date of First Admission to Facility: __/__/____ *Date of Current Admission to Facility: __/ __/____
*Event Type: UTI *Date of Event : __/__/____
*Resident Car e Location: _________________________ _
*Primary Res ident Service Type: (check one)
□ Long-term gen eral nursing □ Long-term dement ia □ Long-term psychi at ric
□ Skilled nursing/Short-term rehab (sub acute) □ Ventilator □ Bariatric □ Hospice/Palliative
*Has resident been transferr ed from an acut e c are facilit y to your facility in the past 4 weeks?
□ Yes □ No
If Yes, date of last transfer from acute car e to your facility: __/__/___ _
If Yes, did the resident have an indwelling ur inary catheter at the time of transfer to your f ac i li ty?
□ Yes □ No
*Indwelling Ur i nar y Cathet er s tatus at time of event onset (check one):
□ In place □ Removed within last 2 calendar days □ Not in place
If indwelling urinary catheter status in plac e or removed within last 2 calendar days:
Site where indwelling urinary catheter
Inserted (check one):
□ Your facility □ Acute care hospital □ Other □ Unknown
Date of ind wel l ing urinary catheter Insertion: ___/___/_____
If indwellin g urinary catheter not in place, was another uri nary device type present at the time of event o ns et?
□ Yes □ No
If Yes, other device type:
□ Suprapubic □ Condom (males only) □ Intermittent straight catheter
Event Details
*Specify Criteria Used: (check all that apply)
Laboratory & Diagnostic Testing Signs & Symptoms
□ Fever: Single temperature ≥ 37.8°C (>100°F), or > 37.2°C (>99°F) on
repeated occ asions, or an increase of >1.1°C (>2°F) over baseline
□ Specimen collec ted from cl ean c atch voided urine and
positive culture with ≥ 10
5
CFU/ml of no more than 2
species of microorganisms
□ Rigors □ New onset h ypotension
□ New onset confusion/ func t ion al decli ne □ Specimen collec ted from in/out straight catheter and
positive culture with ≥ 10
2
CFU/ml of any
microorganisms
□ Acute pain, swelling, or t enderness of the testes, epididy mis, or
prostate
□ Acute dysuria □ Purulent drainage at catheter insertion si te □ Specimen collec ted from indwelling catheter and
positive culture with ≥ 10
5
CFU/ml of any
microorganisms
New and/or marked in cr ease in (check all that apply):
□ Urgency □ Costovertebral angle pain or tenderness □ Leukocytosis (> 14,000 cells/mm
3
), or Left shift (> 6% or
1,500 bands/mm
3
)
□ Frequency □ Suprapubic tender nes s
□ Incontinence □ Visible (gross) hematuria
□ Positive blood culture with 1 matching organis m in urine
culture
*Specific Ev ent (Check one):
□ Symptomatic UTI (SUTI) □ Symptomatic C A-UTI (CA-SUTI) □ Asymptomatic Bacteremic UTI (ABUTI)
Secondary Bl oodstream Infection: Yes No Died within 7 days of date of ev ent: Yes No
*Transfer to acute care facility within 7 days: Yes No
*Pathogens identified: Yes No *If Yes, speci fy on page 2
Assurance of Confidentiality: The voluntarily provided information obtained in this surveillance system that would permit identification of any individual or institution is collected with a guarantee that it will
be held in strict confidence, will be used only for the purposes stated, and will not otherwise be disclosed or released without the consent of the individual, or the institution in accordance with Sections
304, 306 and 308(d) of the Public Health Service Act (42 USC 242b, 242k, and 242m(d)).
Public reporting burden of this collection of informati on is est im at e d to av erage 30 minutes per response, including the time for reviewing instructions, searching existing data sources, gathering and
maintaining the data needed, and completing and reviewing the collection of information. An agency may not conduct or sponsor, and a person is not required to respond to a collection of information
unless it displays a currently valid OMB control number. Send comments regarding this burden estimate or any other aspect of this collection of information, including suggestions for reducing this burden
to CDC, Reports Clearance Officer, 1600 Clifton Rd., MS D-74, Atlanta, GA 30333, ATTN: PRA (0920-0666).
CDC 57.140 (Front) v8.3
Form Approved
OMB No. 0920-0666
Exp. Date: 11/30/2019
www.cdc.gov/nhsn
CDC 57.140 (Back), r1 v8.3
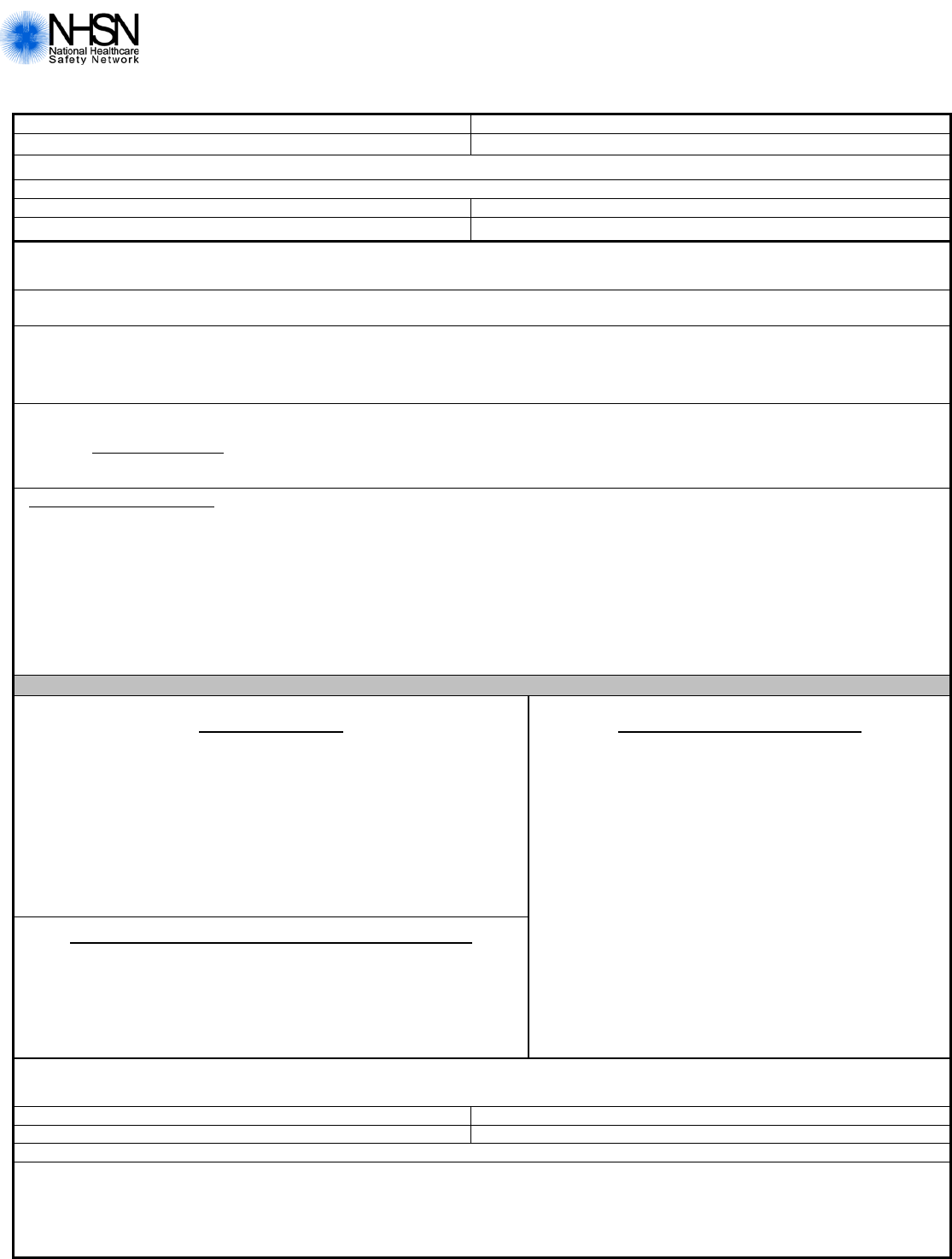
Urinary Tract Infection (UTI) for LTCF
Page 2 of 4
Pathogen
#
Gram-positive Organisms
_______
Staphylococcus coagulase-negative
VANC
S I R N
(specify species if available):
____________
_______
____Enterococ cus faecium
____Enterococ cus faecalis
____Enterococcus spp.
(Only those not identified to the
species level)
DAPTO
S NS N
GENTHL
§
S R N
LNZ
S I R N
VANC
S I R N
_______
Staphylococcus
aureus
CIPRO/LEVO/MOXI
S I R N
CLIND
S I R N
DAPTO
S NS N
DOXY/MINO
S I R N
ERYTH
S I R N
GENT
S I R N
LNZ
S R N
OX/CEFOX/METH
S I R N
RIF
S I R N
TETRA
S I R N
TIG
S NS N
TMZ
S I R N
VANC
S I R N
Pathogen
#
Gram-negative Organisms
_______
Acinetobacter
(specify species)
____________
AMK
S I R N
AMPSUL
S I R N
AZT
S I R N
CEFEP
S I R N
CEFTAZ
S I R N
CIPRO/LEVO
S I R N
COL/PB
S I R N
GENT
S I R N
IMI
S I R N
MERO/DORI
S I R N
PIP/PIPTAZ
S I R N
TETRA/DOXY/MINO
S I R N
TMZ
S I R N
TOBRA
S I R N
_______
Escherichia coli
AMK
S I R N
AMP
S I R N
AMPSUL/AMXCLV
S I R N
AZT
S I R N
CEFAZ
S I R N
CEFEP
S I/S-DD R N
CEFOT/CEFTRX
S I R N
CEFTAZ
S I R N
CEFUR
S I R N
CEFOX/CETET
S I R N
CIPRO/LEVO/MOXI
S I R N
COL/PB
†
S R N
ERTA
S I R N
GENT
S I R N
IMI
S I R N
MERO/DORI
S I R N
PIPTAZ
S I R N
TETRA/DOXY/MINO
S I R N
TIG
S I R N
TMZ
S I R N
TOBRA
S I R N
_______
Enterobacter
(specify species)
____________
AMK
S I R N
AMP
S I R N
AMPSUL/AMXCLV
S I R N
AZT
S I R N
CEFAZ
S I R N
CEFEP
S I/S-DD R N
CEFOT/CEFTRX
S I R N
CEFTAZ
S I R N
CEFUR
S I R N
CEFOX/CETET
S I R N
CIPRO/LEVO/MOXI
S I R N
COL/PB
†
S R N
ERTA
S I R N
GENT
S I R N
IMI
S I R N
MERO/DORI
S I R N
PIPTAZ
S I R N
TETRA/DOXY/MINO
S I R N
TIG
S I R N
TMZ
S I R N
TOBRA
S I R N
_______
____Klebsiella
pneumonia
____Klebsiella
oxytoca
AMK
S I R N
AMP
S I R N
AMPSUL/AMXCLV
S I R N
AZT
S I R N
CEFAZ
S I R N
CEFEP
S I/S-DD R N
CEFOT/CEFTRX
S I R N
CEFTAZ
S I R N
CEFUR
S I R N
CEFOX/CETET
S I R N
CIPRO/LEVO/MOXI
S I R N
COL/PB
†
S R N
ERTA
S I R N
GENT
S I R N
IMI
S I R N
MERO/DORI
S I R N
PIPTAZ
S I R N
TETRA/DOXY/MINO
S I R N
TIG
S I R N
TMZ
S I R N
TOBRA
S I R N
Form Approved
OMB No. 0920-0666
Exp. Date: 11/30/2019
www.cdc.gov/nhsn
CDC 57.140 (Back), r1 v8.3
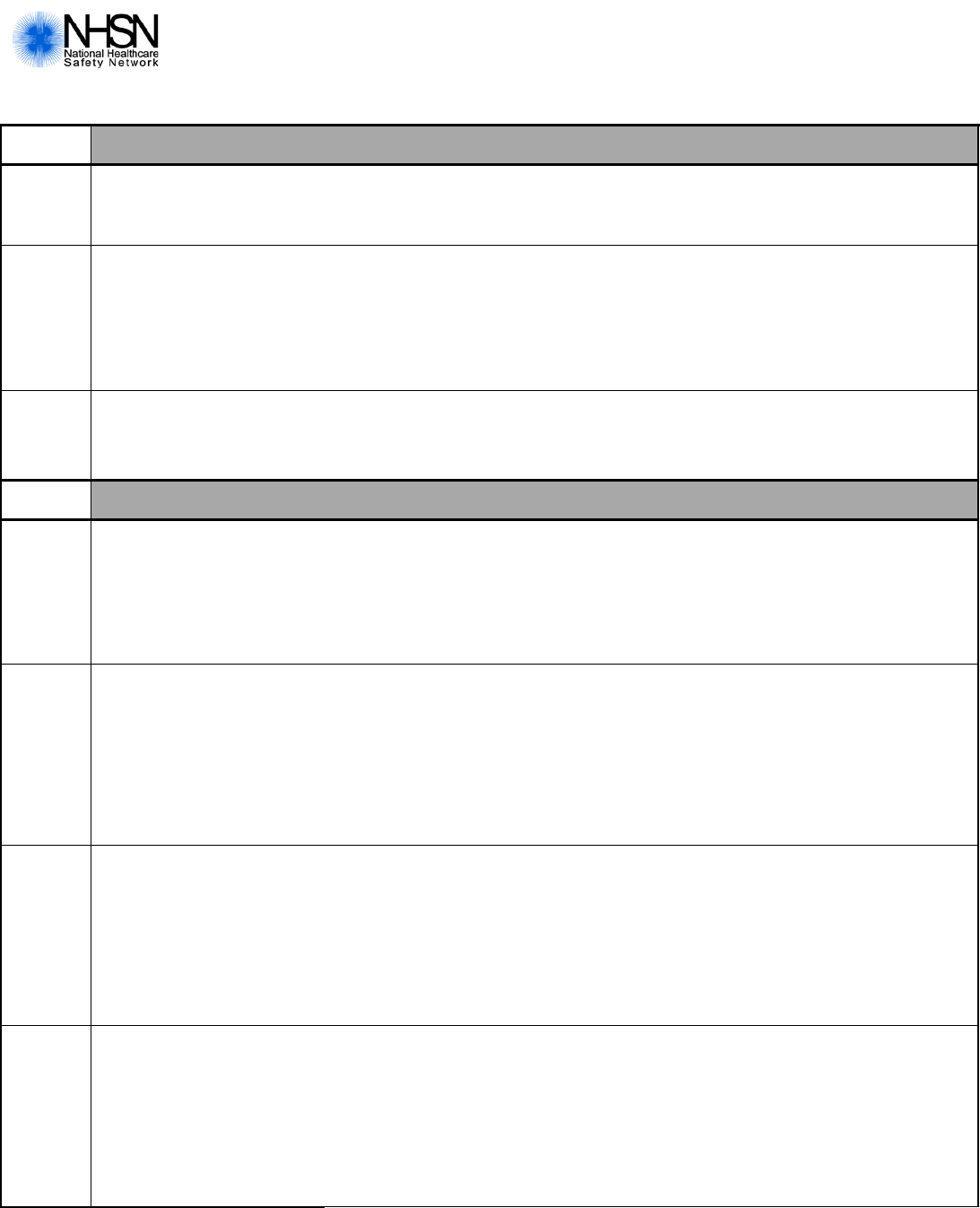
Urinary Tract Infection (UTI) for LTCF
Page 3 of 4
Pathogen
#
Gram-negative Organisms (continued)
_______
Pseudomonas
aeruginosa
AMK
S I R N
AZT
S I R N
CEFEP
S I R N
CEFTAZ
S I R N
CIPRO/LEVO
S I R N
COL/PB
S I R N
GENT
S I R N
IMI
S I R N
MERO/DORI
S I R N
PIP/PIPTAZ
S I R N
TOBRA
S I R N
Pathogen
#
Fungal Organisms
_______
Candida
(specify species if
available)
____________
ANID
S I R N
CASPO
S NS N
FLUCO
S S-DD R N
FLUCY
S I R N
ITRA
S S-DD R N
MICA
S NS N
VORI
S S-DD R N
Pathogen
#
Other Organisms
_______
Organism 1
(specify)
____________
_______
Drug 1
S I R N
_______
Drug 2
S I R N
______
Drug 3
S I R N
_______
Drug 4
S I R N
_______
Drug 5
S I R N
______
Drug 6
S I R N
______
Drug 7
S I R N
______
Drug 8
S I R N
______
Drug 9
S I R N
_______
Organism 1
(specify)
____________
_______
Drug 1
S I R N
_______
Drug 2
S I R N
______
Drug 3
S I R N
_______
Drug 4
S I R N
_______
Drug 5
S I R N
______
Drug 6
S I R N
______
Drug 7
S I R N
______
Drug 8
S I R N
______
Drug 9
S I R N
_______
Organism 1
(specify)
____________
_______
Drug 1
S I R N
_______
Drug 2
S I R N
______
Drug 3
S I R N
_______
Drug 4
S I R N
_______
Drug 5
S I R N
______
Drug 6
S I R N
______
Drug 7
S I R N
______
Drug 8
S I R N
______
Drug 9
S I R N
Result Cod es
S = Susceptible I = Intermediate R = Resistant NS = Non-susceptib le S-DD = Susceptible-dose dependen t N = Not tested
§
GENTHL results: S = Suscept ible/Synerg istic and R = Resist ant/Not Synergistic
†
Clinical breakpoints have not been set by FDA or CLSI, Sensitive and Resistant designations should be based upon
epidemiological cutoffs of Sensitive MIC ≤ 2 and Resistant MIC ≥ 4
Drug Codes:
AMK = amikacin CEFTRX = ceftriaxone FLUCY = flucytosine OX = oxacillin
AMP = ampicillin CEFUR= cefuroxime GENT = gentamicin PB = polymyxin B
AMPSUL = ampicillin/sulbactam CETET= cefotetan
GENTHL = gentamicin –high level
test
PIP = piperacillin
AMXCLV = amoxicillin/clavulanic acid CIPRO = ciprofloxacin IMI = imipenem PIPTAZ = piperacillin/tazobactam
ANID = anidulafungin CLIND = clindamycin ITRA = itraconazole RIF = rifampin
AZT = aztreonam COL = colistin LEVO = levofloxacin TETRA = tetracycline
CASPO = caspofungin DAPTO = daptomycin LNZ = linezolid TIG = tigecycline
CEFAZ= cefazolin DORI = doripenem MERO = meropenem
TMZ =
trimethoprim/sulfamethoxazole
CEFEP = cefepime DOXY = doxycycline METH = methicillin TOBRA = tobramycin
CEFOT = cefotaxime ERTA = ertapenem MICA = micafungin VANC = vancomycin
CEFOX= cefoxitin ERYTH = erythromycin MINO = minocycline VORI = voriconazole
CEFTAZ = ceftazidime FLUCO = fluconazole MOXI = moxifloxacin
Form Approved
OMB No. 0920-0666
Exp. Date: 11/30/2019
www.cdc.gov/nhsn
CDC 57.140 (Back), r1 v8.3
Urinary Tract Infection (UTI) for LTCF
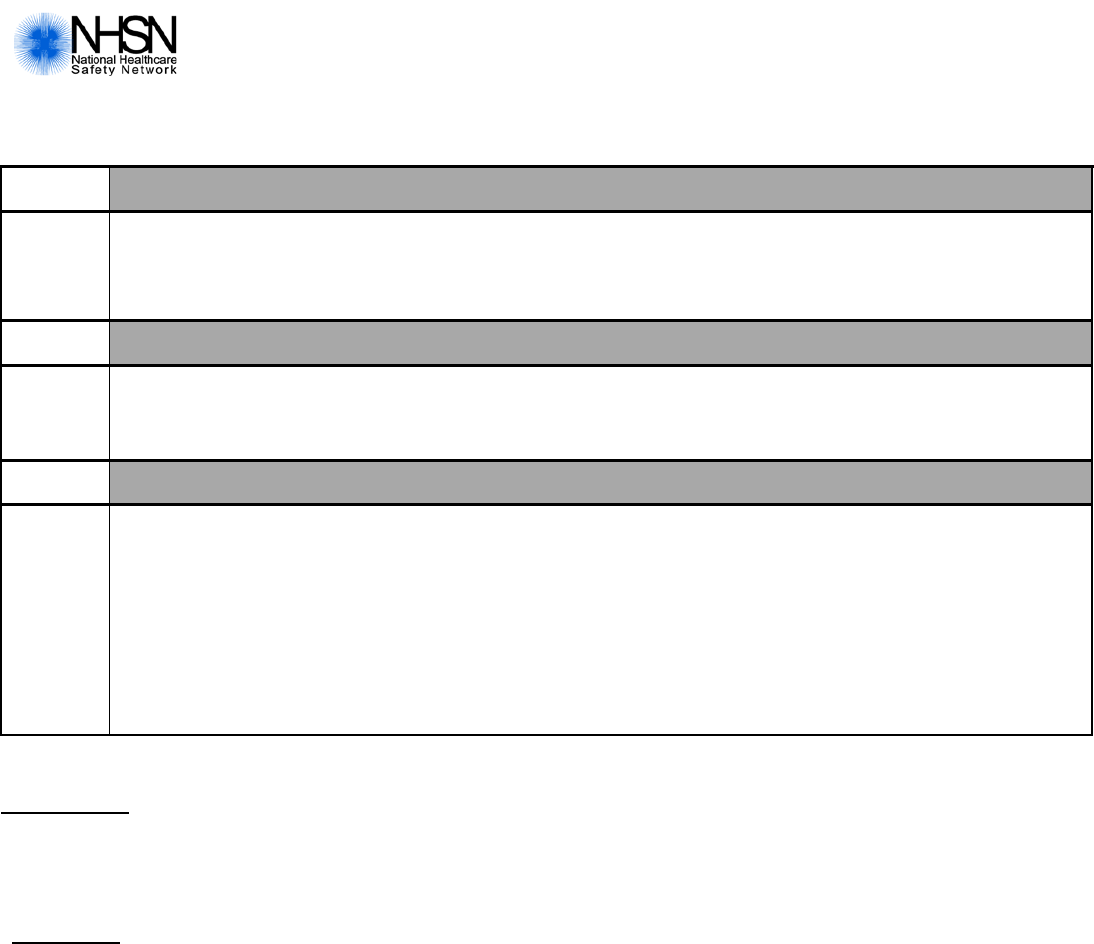
Page 4 of 4
Custom Fields
Label Label
______________________ ____/____/____ _______________________ ____/____/_____
_______________________ _____________ _______________________ ______________
_______________________ _____________ _______________________ ______________
_________________________ ______________ _______________________ ______________
_________________________ ______________ _______________________ ______________
_________________________ ______________ _______________________ ______________
_________________________ ______________ _______________________ ______________
Comments



