Fillable Printable 57.316 Toi
Fillable Printable 57.316 Toi

57.316 Toi
NHSN Biovigilance Component
Tables of Instruction v2.1 (57.316)
www.cdc.gov/nhsn
Page 1 of 7
January 2017
Hemovigilance Module
Adverse Reaction
Transfusion associated graft vs. host disease (TA-GVHD)
Data Field Instructions for Form Completion
Facility ID# The Facility ID number will be auto entered by NHSN.
Adverse Reaction # An adverse reaction number will be auto entered by NHSN.
Patient Information
Patient ID
Required. Enter the medical record number or other facility
alphanumeric identification code for the patient. Note: Facility patient
information is shared across NHSN Component. When an MRN is
entered for a patient that has been previously entered for another
NHSN event, the patient information will automatically populate.
NHSN is HIPPA compliant; it is not recommended to devise a unique
patient identifier for NHSN.
Gender
Required. Select the gender of the transfusion recipient.
Date of birth
Required. Enter the date of birth of the transfusion recipient.
Social Security # Optional. For local use only.
Secondary ID Optional. For local use only.
Medicare # Optional. For local use only.
Last Name Optional. For local use only.
First Name Optional. For local use only.
Middle Name Optional. For local use only.
Ethnicity Optional. For local use only.
Race Optional. For local use only.
Blood group
Required. Select the blood group of the transfusion recipient. Note:
If the patient’s blood type does not clearly match a single blood type,
select the most relevant blood type and make a note in the
comments section of the form. For example, if a patient is typing with
mixed field reactions following a bone marrow transplant, select the
predominant blood type and enter a note in the comments section
such as, “Group A recipient of group O bone marrow transplant
currently typing as mixed field.”
Patient Medical History
List the patient’s admitting
diagnosis.
Required. Indicate the patient’s admitting diagnosis. NOTE: For
more information about the Patient Medical History question, please
refer to the Patient Medical History QuickLearn on the NHSN Blood
Safety Surveillance website.
Code:
Indicate the International Classification of Diseases (ICD) -10-CM
code for the patient’s admitting diagnosis.
NHSN Biovigilance Component
Tables of Instruction v2.1 (57.316)
www.cdc.gov/nhsn
Page 2 of 7
January 2017
Data Field Instructions for Form Completion
Description:
Indicate the International Classification of Diseases (ICD) -10-CM
description for the patient’s admitting diagnosis.
List the patient’s underlying
indication for transfusion.
(Use ICD-10 Diagnostic
codes/descriptions)
Required. Indicate the patient’s underlying indication for transfusion..
NOTE: For more information about the Patient Medical History
question, please refer to the Patient Medical History QuickLearn on
the NHSN Blood Safety Surveillance website.
Code:
Indicate the International Classification of Diseases (ICD) -10-CM
code for the patient’s underlying indication for transfusion.
Description:
Indicate the International Classification of Diseases (ICD) -10-CM
description for the patient’s underlying indication for transfusion.
List the patient’s comorbid
conditions at the time of the
transfusion related to the
adverse reaction. (Use
ICD-10 Diagnostic
codes/descriptions)
Required. Indicate the patient’s comorbid conditions at the time of the
transfusion related to the adverse reaction. NOTE: For more
information about the Patient Medical History question, please refer
to the Patient Medical History QuickLearn on the NHSN Blood Safety
Surveillance website.
Code:
Indicate the International Classification of Diseases (ICD) -10-CM
code for the patient’s comorbid conditions at the time of the transfusion
related to the adverse reaction.
Description:
Indicate the International Classification of Diseases (ICD) -10-CM
description for the patient’s comorbid conditions at the time of the
transfusion related to the adverse reaction.
UNKNOWN
Check box if the patient’s comorbid conditions at the time of the
transfusion related to the adverse reaction are unknown.
NONE
Check box if the patient has NO comorbid conditions at the time of the
transfusion related to the adverse reaction.
List the patient’s relevant
medical procedure
including past procedures
and procedures to be
performed during the
current hospital or
outpatient stay. (Use ICD-
10 Procedure
codes/descriptions)
Required. Indicate the patient’s relevant medical procedure including
past procedures and procedures to be performed during the current
hospital or outpatient stay. NOTE: For more information about the
Patient Medical History question, please refer to the Patient Medical
History QuickLearn on the NHSN Blood Safety Surveillance website.
Code:
Indicate the International Classification of Diseases (ICD) -10-CM
code for the patient’s relevant medical procedure including past
procedures and procedures to be performed during the current hospital
or outpatient stay.
Description:
Indicate the International Classification of Diseases (ICD) -10-CM
description for the patient’s relevant medical procedure including past
procedures and procedures to be performed during the current hospital
or outpatient stay.
UNKNOWN
Check box if the patient’s relevant medical procedure including past
procedures and procedures to be performed during the current hospital
or outpatient stay are unknown.
NHSN Biovigilance Component
Tables of Instruction v2.1 (57.316)
www.cdc.gov/nhsn
Page 3 of 7
January 2017
Data Field Instructions for Form Completion
NONE
Check box if the patient has NO relevant medical procedure including
past procedures and procedures to be performed during the current
hospital or outpatient stay.
Additional Information
Optional. Include additional information related to the patient’s
medical history not included in the previous questions.
Transfusion History
Has the patient received a
previous transfusion?
Required. Indicate if the patient experienced an adverse reaction
during a previous transfusion that is related to the current adverse
reaction event being reported.
If yes, provide information
about the transfusion
event.
Conditionally required. If the patient received a previous transfusion,
complete the next section. If not, skip to Reaction Details section.
Blood Product:
Conditionally required. Indicate the previously transfused blood
product.
Date of Transfusion:
Conditionally required. Indicate the date of the previous transfusion.
Did the patient
experience a transfusion
adverse reaction?
Conditionally required. Indicate whether the patient experienced a
transfusion adverse reaction related to the previous transfusion.
Type of transfusion
adverse reaction:
Conditionally required. Complete if the patient experienced a
transfusion adverse reaction. Indicate the type of transfusion adverse
reaction.
Specify
Conditionally required. Complete if the patient experienced an
“Other” transfusion adverse reaction. Specify the transfusion adverse
reaction. Note: Use this option if the recipient was diagnosed with an
adverse reaction that is not defined in the Hemovigilance Module
protocol (e.g., transfusion-associated acute gut injury (TRAGI),
thrombosis).
Reaction Details
Date reaction occurred Required. Enter the date the reaction was first observed in the
transfusion recipient.
Time reaction occurred Required. Enter the time the reaction was first observed in the
transfusion recipient using a 24-hour clock.
Facility location where
patient was transfused
Required. Select the facility location where the patient was
transfused. Note: Only report reactions for recipients transfused by
your facility.
Link/Unlink Incidents Conditionally required. Select associated incidents from the list
populated by NHSN and SAVE. Note: The incident record must be
entered into the system first and must include the associated Patient
ID number(s). When linking the adverse reaction record, NHSN
searches for matching Patient ID numbers in the incident records.
After recognition of the
transfusion reaction, was
the current transfusion:
Conditionally required. Indicate what action was taken with the blood
product after the transfusion adverse reaction was recognized.
Investigation Results
NHSN Biovigilance Component
Tables of Instruction v2.1 (57.316)
www.cdc.gov/nhsn
Page 4 of 7
January 2017
Data Field Instructions for Form Completion
Transfusion associated
graft vs. host disease (TA-
GVHD)
Required. Using the case definition criteria in Section 3 of the
Hemovigilance Module surveillance protocol, select the adverse
reaction being reported. Check the box if you are reporting
Transfusion associated graft vs. host disease (TA-GVHD).. Proceed
with the next question. If you are reporting a different type of
transfusion reaction, STOP. Select the form for the correct type of
transfusion reaction. Note: Report only one adverse reaction per
form. Report the reaction after the investigation has been
finalized. Incomplete records cannot be saved. If new information
becomes available at a later time, the record can be edited.
Case definition
Required. Using the case definition criteria in Section 3 of the
Hemovigilance Module surveillance protocol, select the case criteria
met for the reported adverse reaction.
Did patient receive non-
irradiated blood
product(s) in the two
months preceding the
reaction?
Required. Specify whether the patient received any non-irradiated
blood products in the two months prior to the TAGVHD reaction.
Check all that occurred
within 2 days to 6
weeks after cessation
of transfusion:
Required. Check all signs and symptoms observed in the patient at
the time the reaction occurred as well as any associated laboratory
findings. See Section 3 in the Hemovigilance Module surveillance
protocol for a glossary of signs and symptoms.
Check all that apply:
Required. Check all conditions that that apply to the reaction or the
patient.
Other signs and symptoms
Required. Check all additional signs and symptoms observed in the
patient at the time the reaction occurred as well as any other
associated findings.
Severity
Required. Using the case definition criteria in Section 3 of the
Hemovigilance Module surveillance protocol, select the severity
criteria met for the reported adverse reaction.
Did the patient receive
or experience any of
the following?
Required. Check all options that apply. See Section 3 in the
Hemovigilance Module surveillance protocol for severity definitions.
Imputability
Required. Using the case definition criteria in Section 3 of the
Hemovigilance Module surveillance protocol, select the imputability
criteria met for the reported adverse reaction. Note: Doubtful and
Ruled Out need not be routinely reported.
Which best describes
the relationship
between the transfusion
and the reaction?
Required. Check ONE option that best describes the relationship
between the transfusion and the reaction. See Section 3 in the
Hemovigilance Module surveillance protocol for imputability
definitions.
Did the transfusion
occurred at your
facility?
Required. Indicate whether the transfusion that likely caused the
transfusion reaction occurred at your facility.
NHSN Biovigilance Component
Tables of Instruction v2.1 (57.316)
www.cdc.gov/nhsn
Page 5 of 7
January 2017
Data Field Instructions for Form Completion
WBC chimerism:
Required. Indicate whether a WBC chimerism is present If a WBC
chimerism test was performed. If a WBC chimerism is NOT present
or the test, check the appropriate box.
Additional Information Optional. Provide any additional relevant information.
Outcome
Outcome
Required. Enter the outcome of the transfusion recipient.
Date of death Conditionally required. If the recipient died following the adverse
reaction, enter the date of death whether or not the death was
transfusion related.
Relationship of
transfusion to death
Conditionally required. If the recipient died following the adverse
transfusion reaction, indicate the relationship of the transfusion to
death using the imputability criteria for “Other/Unknown” adverse
reactions defined in Section 3 of the Hemovigilance Module
surveillance protocol.
Cause of death:
Conditionally required. Indicate the cause of death.
Was an autopsy
performed?
Conditionally required. Indicate whether an autopsy was performed.
Patient Treatment
Did the patient receive
treatment for the
transfusion reaction?
Required. Indicate whether the patient received treatment for the
transfusion adverse reaction. If the patient received treatment,
complete the following section. If not, skip to the component details
section.
Select treatment(s):
Conditionally required. Indicate the type of treatment provided in
response to the transfusion adverse reaction. Select all that apply.
Select type of
medication(s),
respiratory support, or
renal replacement
therapy
Conditionally required. Complete if patient received medication(s),
respiratory support, or renal replacement therapy. Select the type of
medication(s), respiratory support, or renal replacement therapy.
Other, Specify
Conditionally required. Complete if patient received another type of
treatment not listed above. Specify the type of treatment.
Component Details
Was a particular unit
implicated in (i.e.,
responsible for) the
adverse reaction?
Required. Indicate whether or not a specific unit could be identified
as the likely cause of the adverse reaction. Details for the implicated
unit must be entered on the first row of the “Component Details”
table. Determine “implicated” independent of case definition and
imputability criteria. If only one unit was transfused, that unit must be
implicated in the reaction. If TACO is being reported, no specific unit
may be implicated regardless of the number of units transfused.
Transfusion Start Date Required. Enter the date the transfusion started.
Transfusion Start Time Required. Enter the time the transfusion started using a 24-hour
clock.
Transfusion End Date Required. Enter the date the transfusion ended.

NHSN Biovigilance Component
Tables of Instruction v2.1 (57.316)
www.cdc.gov/nhsn
Page 6 of 7
January 2017
Data Field Instructions for Form Completion
Transfusion End Time Required. Enter the time the transfusion ended using a 24-hour
clock.
Component code (check
system used)
Required. Select the labeling system used for the transfused
component(s). Select Other to list a local blood product code. Note:
Codabar- and ISBT 128-labeled products may be entered, but
each must be entered on their own row.
Component code
(___ ___ ___ ___ ___)
Required. Enter the component code for the product transfused
using only the portion that identifies the product type. In the sample
label below, the code that identifies the product type is 04250.
Note: Enter all components
administered within 24 hours prior to an acute transfusion reaction.
Enter only the component(s) most likely responsible for delayed
reactions based on temporal relationship and clinical judgment.
Note: If the code entered does not match a product description in
NHSN, “Component code not found” will appear in the product
description field. Verify your data entry before continuing; an
incorrect or unrecognized component code will not prevent you from
saving the adverse reaction record.
Blood collection
establishment
Conditionally required. Complete if Codabar component code was
entered above. Indicate the blood collection establishment that
collected the blood product.
Amount transfused at
reaction onset
Required. Indicate the amount transfusion at reaction onset.
Entire unit Select if the entire unit was transfused at reaction onset.
Partial unit Select if only part of the unit was transfused at reaction onset.
Volume transfused
_______mL
Complete if a partial unit was transfused. Indicate the volume
transfused at reaction onset.
Unit number
Required. For all reaction types, enter the individual unit number as
it appears on the product label. Unit number is optional for all other
adverse reactions.
The sample ISBT-128 unit number would be entered as seen below.
W 0 0 0 0
0 7
1 2 3 4 5 6
0 0
D
Note: The check digit is optional. If the check digit is entered, the
system will verify that it is correct using an internal check digit
NHSN Biovigilance Component
Tables of Instruction v2.1 (57.316)
www.cdc.gov/nhsn
Page 7 of 7
January 2017
Data Field Instructions for Form Completion
calculator. If the check digit is not entered, the space will remain
blank.
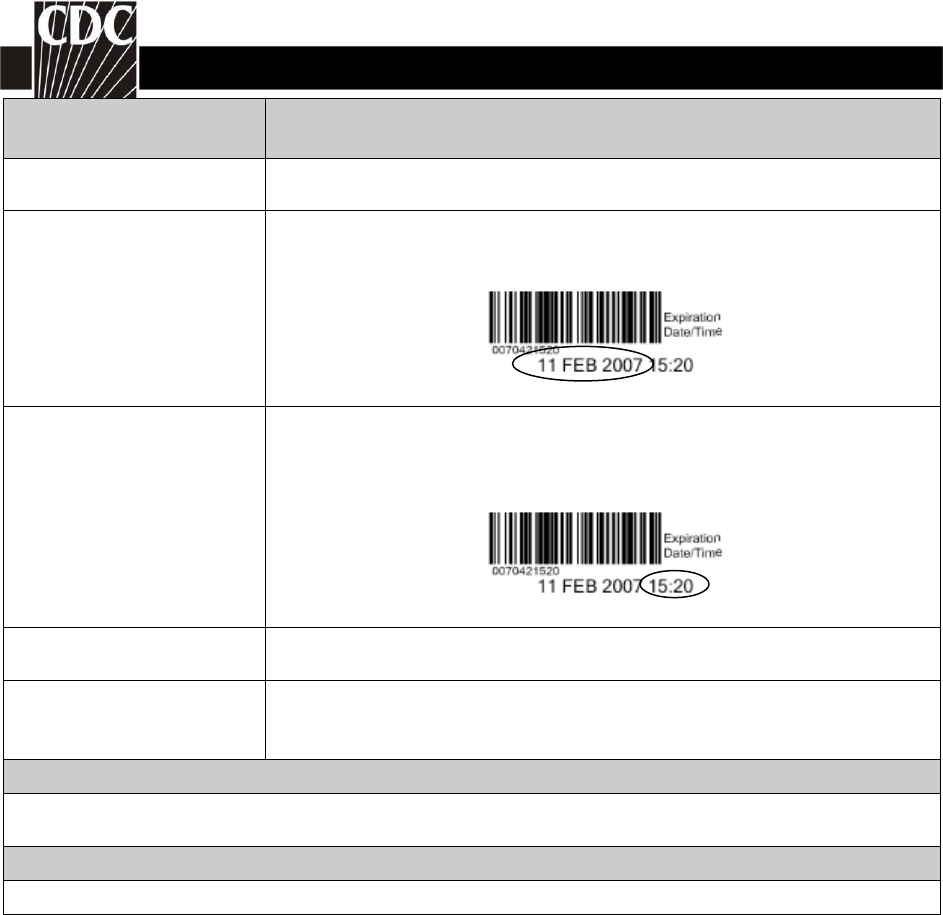
Unit expiration date
Required. Enter the expiration date of the unit(s). The expiration
date for the sample label below would be 02/11/2007.
Unit expiration time Required. Enter the expiration time of the unit(s). NHSN will auto fill
this editable field to 23:59(11:59PM). The expiration time for the
sample label below would be 15:20.
Blood group of unit
Required. Select the blood group of the unit(s) transfused; enter N/A
for products where blood group is not applicable.
Implicated in the adverse
reaction?
Conditionally required. If a particular unit was implicated, the unit
details must be entered on the first row and this box will be checked.
If no unit can be implicated, these boxes will be inactive.
Custom Fields
Optional. Up to 50 custom fields may be added to this form for local use. Custom data may be
collected in an alphanumeric, numeric, or date format.
Comments
Optional. Enter additional information about the incident.



