Fillable Printable Research Report Template - USAID Learning Lab
Fillable Printable Research Report Template - USAID Learning Lab
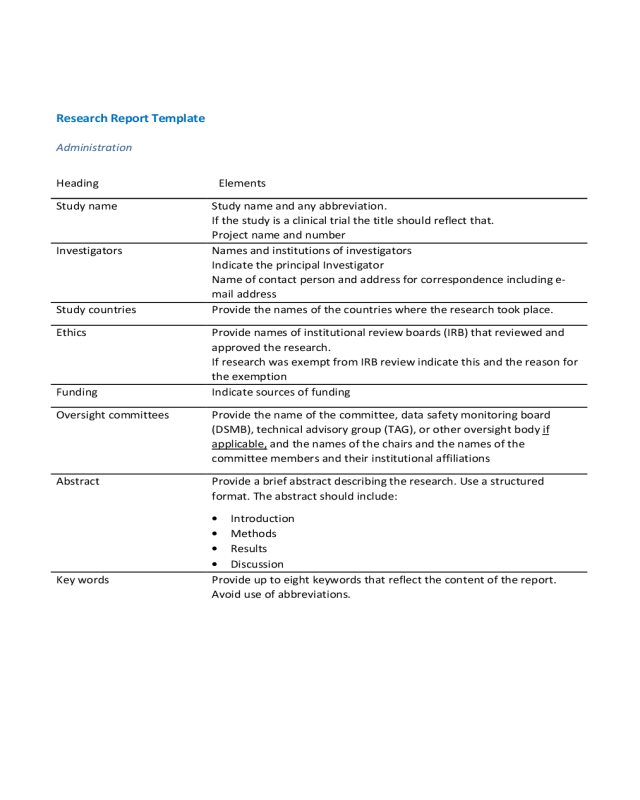
Research Report Template - USAID Learning Lab
Research Report Template
Administration
Heading Elements
Study name Study name and any abbreviation.
If the study is a clinical trial the title should reflect that.
Project name and number
Investigators Names and institutions of investigators
Indicate the principal Investigator
Name of contact person and address for correspondence including e-
mail address
Study countries Provide the names of the countries where the research took place.
Ethics Provide names of institutional review boards (IRB) that reviewed and
approved the research.
If research was exempt from IRB review indicate this and the reason for
the exemption
Funding Indicate sources of funding
Oversight committees Provide the name of the committee, data safety monitoring board
(DSMB), technical advisory group (TAG), or other oversight body if
applicable, and the names of the chairs and the names of the
committee members and their institutional affiliations
Abstract Provide a brief abstract describing the research. Use a structured
format. The abstract should include:
Introduction
Methods
Results
Discussion
Key words Provide up to eight keywords that reflect the content of the report.
Avoid use of abbreviations.

Problem Formulation
Heading Elements
Background/introduction Provide a concise statement of the purpose and scope of the study.
Explain why the topic is important if this is not obvious.
Summarize previous research on the topic. Include citations
of both published and gray literature.
Explain why previous work is not sufficient. Indicate how
this study fills gaps in existing knowledge, addresses
unresolved issues, overcomes shortcomings of previous
research or contributes to new knowledge.
If appropriate, provide the conceptual framework or
theoretical underpinnings of the study and rationale for it.
If appropriate, provide the rationale for the methodological
orientation of the study.
If appropriate, provide the rationale for the groups studied.
Describe the context of the study in detail to provide
information relevant to how the findings may or may not be
applicable to other settings.
Objectives Primary study objective, question, or hypothesis
Secondary study objective(s), question(s), or hypothesis(es)
Methods
Heading Elements
Design • Describe the study design and comparison groups (if any).
Sampling •
Describe the population under study and the rationale for
choosing this population if not obvious.
•
Describe the sampling frame used.
• Describe the type of sample drawn (e.g. simple random,
stratified, systematic, probability-proportional-to-size,
purposeful, snowball, etc.), the methodology used, and the
rationale.
•
Describe how the sample size needed for statistical testing
was calculated. Include power calculations.
• Describe any non-participation or non-inclusion among
sample approached and document reasons.
Sources of data and
methods of data
collection
•
Describe all sources of data used.
• Describe how instruments were developed and attach all
instruments used in data collection.
•
Describe the unit(s) of analysis.
• Describe in detail how, where, when and by whom data
were collected and how field settings or timing may have
influenced data collected in that context.
Variables and measures •
Describe any standard measures or instruments used and
provide information on their reliability and validity.
•
If new measures or indexes were developed (e.g. through
data reduction techniques), sufficient detail should be
provided to make clear how the variables were constructed
and how their reliability and validity was assessed.
• When transcripts of recordings or notes are used, the
method to classify/code response categories or
characterize actions should be clearly described.
Data analysis • Describe the procedures used for analysis including
software packages.
•
Describe the analytic techniques/test statistics used.
• For qualitative studies, describe how data were organized
into analytic categories and how any constructed analytic
concepts/domains have been used, if applicable.

Results and Conclusions
Heading Elements
Issues in data collection • Disclose any issues that arose in data collection
and processing (e.g. missing data, losses to follow
up, violations of statistical assumptions, possible
sources of bias etc.) and how these were handled
in cleaning and data analysis.
• Describe the quality of data sources such as clinic
records and the context of their use.
Presentation of results • Use tables and graphs to summarize information.
In general, tables are better than graphs for giving
structured numeric information, whereas graphs
are better for indicating trends and making broad
comparisons or showing relationships. Tables and
graphs should, ideally, be self-explanatory. The
reader should be able to understand them
without detailed reference to the text. The title
should be informative, and rows and columns of
tables or axes of graphs should be clearly labeled.
The source of data should be given at the bottom.
• For each statistical result there should be included
a measure of the relation between variables (e.g.
odds ratio, regression coefficient), an index of
uncertainty (e.g. confidence interval), and a
qualitative judgment as to the importance of the
finding.
• For qualitative analyses, describe the processes
throughout data collection of identifying
categories of events, actions, subgroups of
people, or other substantive categories by which
data were organized and patterns of observations
identified. The process of developing descriptions,
claims and interpretations should be clearly
described and illustrated. Evidence to support
each claim should be presented. Practices used to
develop and enhance the evidence for each claim
should be described including the search for
disconfirming evidence and alternative
interpretations. Interpretive commentary should
provide a deeper understanding of the claims –
e.g. how and why the patterns described may
have occurred; how they relate to one another;
how they support or challenge theory and findings
from previous research. Use direct quotations
from informants to illustrate points made.
• Report unexpected findings and how that affected
analysis.
Presentation of conclusions •
Summarize the main findings and their
interpretation, clearly linking them to the
purpose/hypothesis of the study presented
above.
• Indicate alternative explanations for the findings
and any possible sources of bias.
• Indicate to whom the results in this context may
be generalized, or the limits to generalization.
•
Discuss how the results might be applied in
practice as well as the policy and program
implications.
Ethical considerations •
State which institutional review boards approved
the study or if the study was deemed exempt.
• Report research results in a way that honors
consent agreements with human subjects and any
other agreements with respect to gaining access
to research sites, data or materials.
•
Include statements about potential conflicts of
interest.
Dissemination of findings • Discuss how findings were shared with the
research population and other key stakeholders.



